Table 3: Protective Action Criteria (PAC) Rev. 29 Based on Applicable 60-Minute Aegls, Erpgs, Or Teels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

United States Patent Office Patented Sept
3,149,913 United States Patent Office Patented Sept. 22, 1964 2 may vary over a wide range and may be as little as 1% 3,49,913 and as much as 50% and even higher. Particularly ad PROCESS FOR PRODUCING NETROSYL vantageous is the use of nitric acid in amounts such that SULFURECACE) d the resulting nitrosylsulfuric acid concentration approxi ALouis L. Ferstandig, El Cerrito, and Paul C. Condit, San 5 mates saturation values in order to afford maximum pro Asseino,poration, Calif.,San Francisco,assignors toCalif., California a corporation Research Cor of duction per unit reactor volume and yet avoid deposition Beavy are of solids. The solubility of nitrosylsulfuric acid in ap No Drawing. Fied June 14, 1961, Ser. No. 116,957 proximately 100% sulfuric acid ranges from about 48 3 (Caims. (CE. 23-39) grams per 100 grams of solution at 0° C. up to about 68 grams at 50 C. with, of course, a lesser solubility below This invention relates to a proces for the production of O 0 C. and a greater above 50° C. Although the presence nitrosylsulfuric acid. of the precipitated nitrosylsulfuric acid is usually a source Nitrosyisulfuric acid is particularly desirable for use of mechanical inconvenience, advantage may be taken of in the production of caprolactam from hexahydrobenzoic it by removal of the solid nitrosylsulfuric acid by filtra acid. Nitrosylsulfuric acid has long been known as an 5 tion and subsequent recycle of the mother liquor to the intermediate in connection with the lead-chamber sulfuric reaction Zone. acid process in which it is converted to Sulfuric acid with in general, the effective temperature range of the process concurrent liberation of nitric oxide in a reaction with is defined by the requirement that the reaction medium be sulfur dioxide. -

Industry Compliance Programme
Global Chemical Industry Compliance Programme GC-ICP Chemical Weapons Convention December 2006 Version 1.0 GLOBAL CHEMICAL INDUSTRY COMPLIANCE PROGRAMME FOR IMPLEMENTING THE CHEMICAL WEAPONS CONVENTION The purpose of the handbook is to provide guidance to chemical facilities, traders and trading companies in developing a Global Chemical Industry Compliance Programme (GC-ICP) to comply with the Chemical Weapons Convention (CWC). The GC-ICP focuses first on determining if there is a reporting requirement to your National Authority and second on collecting the relevant support data used to complete the required reports. The GC-ICP is designed to provide a methodology to comply with the CWC and establish systems that facilitate and demonstrate such compliance. Each facility/company should also ensure that it follows its country’s CWC specific laws, regulations and reporting requirements. • Sections 2, 3, and 4 guide you through the process of determining if chemicals at your facility/ company should be reported to your National Authority for compliance with the CWC. • Section 5 provides recommended guidance on information that you may use to determine your reporting requirements under the CWC and administrative tools that your facility/company may use to ensure compliance with the CWC. • Section 6 provides a glossary of terms and associated acronyms. • Section 7 provides a listing of all National Authorities by country. CWC Global Chemical Industry Compliance Programme 1 TABLE OF CONTENTS Section 1 Overview What is the Chemical Weapons Convention? -

(PAC) Rev 24 Based on Applicable Aegls, Erpgs, Or Teels (Chemicals Listed by CASRN) PAC Rev 24 – August 2008
Table 3: Protective Action Criteria (PAC) Rev 24 based on applicable AEGLs, ERPGs, or TEELs (Chemicals listed By CASRN) PAC Rev 24 – August 2008 Table 3 presents a listing of chemicals and PAC data based on the Chemical Abstract Service Registry Numbers (CASRNs)1 of the chemicals. Chemicals without an identified CASRN number are issued an identification number, preceded by the letter “z,” for purposes of the PAC data set. The columns presented in Table 3 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this listing by CASRN. Chemical Name The common name of the chemical. CASRN The Chemical Abstract Service Registry Number for this chemical. TEEL-0 This is the threshold concentration below which most people will experience no appreciable risk of health effects. This PAC is always based on TEEL-0 because AEGL-0 or ERPG-0 values do not exist. PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value. PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value. PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value. Units The units for the PAC values (ppm or mg/m3). Additional information on the chemicals presented here is provided in PAC Tables 1, 2, and 4. Table 3, other PAC Tables, introductory/explanatory material (including a glossary of acronyms and abbreviations), definitions of PAC values, and alternative methods of displaying PAC information are available electronically at: http://www.hss.energy.gov/HealthSafety/WSHP/chem_safety/teel.html. -

New Synthesis Routes for Production of Ε-Caprolactam by Beckmann
New synthesis routes for production of ε-caprolactam by Beckmann rearrangement of cyclohexanone oxime and ammoximation of cyclohexanone over different metal incorporated molecular sieves and oxide catalysts Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Anilkumar Mettu aus Guntur/Indien Berichter: Universitätprofessor Dr. Wolfgang F. Hölderich Universitätprofessor Dr. Carsten Bolm Tag der mündlichen Prüfung: 29.01.2009 Diese Dissertation ist auf den Internetseiten der Hochschulbibliothek online verfügbar. Dedicated to my Parents This work reported here has been carried out at the Institute for Chemical Technolgy and Heterogeneous Catalysis der Fakultät für Mathematik, Informatik und Naturwissenschaften in the University of Technology, RWTH Aachen under supervision of Prof. Dr. Wolfgang F. Hölderich between June 2005 and August 2008. ACKNOWLEDGEMENTS I would like to express my deepest sence of gratitude to my supervisor Prof. Dr. rer. nat. W. F. Hölderich for giving me the opportunity to do my doctoral study in his group. His guidance and teaching classes have allowed me to grow and learn my subject during my Ph.d. He has provided many opportunities for me to increase my abilities as a researcher and responsibilities as a team member. I am grateful for the financial support of this work from Sumitomo Chemicals Co., Ltd, Niihama, Japan (Part One) and Uhde Inventa-Fischer GmBH, Berlin (Part Two). Our collaborators at Sumitomo Chemicals Co., Ltd (Dr. C. Stoecker) and Uhde Inventa- Fischer GmBH (Dr. R. Schaller and Dr. A. Pawelski) provided thoughtful guidance and suggestions for each project. -

Spectrophotometric Determination of Thiocyanate In
Investigative Forensic Sciences © All rights are reserved by Buddha D. Paul and Thomas Research Article Open Access Spectrophotometric Determination of Thiocyanate in Human Saliva by a Unique Iodine-Azide-Chromogenic Substrate Reaction and its Application in Distinguishing Tobacco Smokers from Non-Smokers† Buddha D. Paul and Thomas Bosy Division of Forensic Toxicology, Office of the Armed Forces Medical Examiner, Dover AFB, Delaware, USA. Abstract A method to detect thiocyanate (SCN) in human saliva is presented. Thiocyanate concentrations appear to be diagnostic when classifying smokers or non-smokers, and in determining some clinical conditions. The method involves the reaction of SCN with excess iodine and azide, and spectroscopic detection of unreacted iodine by a chromogenic substrate, ABTS. The calibration was linear over the range of 12.5-150 µmol/L (slope = 0.0086 delta-Abs/SCN µmol/L, intercept = -0.0160 delta-Abs, R2 0.9998). The method was applied to analyze 29 saliva specimens. The results were similar to those obtained from a gas chromatography-mass 2 spectrometry method (slope = 0.9595, R 0.9790). Based on Grubbs equation applied to specimens from non-smoking subjects, a threshold concentration of 1100 µmol/L for SCN was determined to distinguish smokers from the non-smokers. The SCN concentrations in 18 out of 20 saliva specimens collected from 2 smokers were above this threshold. The specimens from smokers were also examined for nicotine and cotinine by a GCMS method. While nicotine concentrations were found to vary, the cotinine concentrations remained stable, 134+29 ng/mL. Generally, the presence of nicotine/cotinine in specimens only indicates exposure to tobacco products, but the presence of any of these compounds with elevated SCN, is an indication of smoking. -

Draft Scope of the Risk Evaluation for Triphenyl Phosphate CASRN 115-86-6
EPA Document# EPA-740-D-20-010 April 2020 United States Office of Chemical Safety and Environmental Protection Agency Pollution Prevention Draft Scope of the Risk Evaluation for Triphenyl Phosphate CASRN 115-86-6 April 2020 TABLE OF CONTENTS ACKNOWLEDGEMENTS ......................................................................................................................5 ABBREVIATIONS AND ACRONYMS ..................................................................................................6 EXECUTIVE SUMMARY .......................................................................................................................8 1 INTRODUCTION ............................................................................................................................11 2 SCOPE OF THE EVALUATION ...................................................................................................11 2.1 Reasonably Available Information ..............................................................................................11 Search of Gray Literature ...................................................................................................... 12 Search of Literature from Publicly Available Databases (Peer-Reviewed Literature) .......... 13 Search of TSCA Submissions ................................................................................................ 19 2.2 Conditions of Use ........................................................................................................................19 Categories -

Calarp) Program
California Accidental Release Prevention (CalARP) Program Administering Agency Guidance January 31, 2005 Preface This document provides general guidance to help Administering Agencies (AAs) implement and enforce the California Accidental Release Prevention (CalARP) Program. The intent is to identify the elements of the Program applicable to each regulated business, and assist AAs with oversight of the CalARP Program statutes and regulations. This document is not a substitute for the CalARP Program regulations; it does not impose legally binding requirements. About This Document This document follows the format of the California Code of Regulations, Title 19, Division 2, Chapter 4.5: California Accidental Release Prevention (CalARP) Program. The regulatory sections are presented in parentheses for ease of reference. Acknowledgements The California Emergency Management Agency (Cal EMA) would like to thank the following people for their valuable assistance in the preparation of this document: Howard Wines, Hazardous Materials Specialist, City of Bakersfield Fire Department Robert Distaso P.E., Fire Safety Engineer, Orange County Fire Authority Randall L. Sawyer, Supervisor, Accidental Release Prevention Programs, Contra Costa County Health Services Department Beronia Beniamine, Senior Hazardous Materials Specialist, Stanislaus County Environmental Resources Department Angie Proboszcz, Risk Management Program Coordinator, USEPA Region 9 Jon Christenson, Senior Environmental Health Specialist, Merced County Department of Public Health Teresa -

US5308512.Pdf
|||||||||||||| USOO530852A United States Patent (19) 11 Patent Number: 5,308,512 Stoll et al. 45 Date of Patent: May 3, 1994 54 THIODIGLYCOLALKOXYLATE 4010606 10/1991 Fed. Rep. of Germany. DERIVATIVES, A PROCESS FOR THEIR 0213067 12/1983 Japan. PRODUCTION AND THEIR USE AS FABRIC 153309 3/1984 Japan. SOFTENERS 1097396 1/1968 United Kingdom . (75) Inventors: Gerhard Stoll, Korschenbroich; OTHER PUBLICATIONS Peter Daute, Essen; Ingo Wegener; Trofimov, Zh. Prikl. Khim., vol. 48, pp. 626-628 1975. Faize Berger, both of Duesseldorf, all Vogel & Krissman & Seifen, Öle, Fette, Wachse, vol. of Fed. Rep. of Germany 115, 1989, pp. 3-8 Article Unavailable. (73) Assignee: Henkel Kommanditgesellschaft auf J. Falbe, Surfactants in Consumer Products, Springer, Aktien, Duesseldorf, Fed. Rep. of 1987, pp. 87-90. Germany Vogel & Krussmann, Seifen, Öle, Fette, Wachse, vol. (21) Appl. No.: 961,683 III, 1985, pp. 567-574 1985. Primary Examiner-Paul Lieberman (22) PCT Filed: Jun. 28, 1991 Assistant Examiner-Michael P. Tierney 86) PCT No.: PCT/EP91/01213 Attorney, Agent, or Firm-Ernest G. Szoke; Wayne C. S371 Date: Mar. 5, 1993 Jaeschke; Real J. Grandmaison S 102(e) Date: Mar. 5, 1993 57) ABSTRACT The process of producing alkoxylated thiodiglycol sulf 87). PCT Pub. No.: WO92/00959 oxide derivatives and thiodiglycol sulfone derivatives PCT Pub. Date: Jan. 23, 1992 corresponding to formula I (30) Foreign Application Priority Data Jul. 7, 1990 (DE) Fed. Rep. of Germany ....... 402,694 CH-CH-O-CH-CHR)-o-x, (I) (O)S 51) Int. Cl. ............................................ D06M 10/08 N 52 U.S.C. ...................................... 252/8.7; 252/8.6; CH-CH2-(O-CH-CHR)-O-X 252/8.9; 568/27; 568/28; 554/227; 560/263; 560/264 wherein X1 and X2 may be the same or different and 58 Field of Search ............... -

(From the Department of Physiology, University of Minnesota, Minneapolis) Same Journal. N. K., Kolloid-Z., 1929, 47, 101. the Jo
THE STRUCTURE OF THE COLLODION MEMBRANE AND ITS ELECTRICAL BEHAVIOR II, THE ACTIVATED COLLODION MEMBRANE BY KARL SOLLNER, IRVING ABRAMS, AND CHARLES W. CARR (From the Department of Physiology, University of Minnesota, Minneapolis) (Received for pubfication, March 31, 1941) I In preceding communicationst, 2 we were led to the conclusion that the elec- trochemical activity of collodion membranes, as manifested by concentration potentials, etc., is due principally to acidic impurities. Accordingly, different brands of collodion differ widely as to their activity, the purer brands being less active. The impure (but active) foreign brands of collodion, heretofore generally used by workers in the field of electrochemical membrane investiga- tion, are no longer obtainable. In order to continue our investigation, it became necessary to find methods to produce active collodion membranes at will. The idea of inducing changes in the electrochemical characteristics of mem- branes is not entirely new. Many investigators have activated membranes by the adsorption of proteins, e.g. the proteinized membranes of Loeb. 8 Other investigators use other organic compounds, usually dyestuffs. These may be adsorbed like proteins or they may be dissolved in the collodion solution* pre- vious to casting the membranes. Such membranes are interesting and useful in their own right, but are not altogether satisfactory substitutes for active collodion membranes. They very often show considerable asymmetry; more- over, the dyestuffs so far employed (according to the literature) are slowly re- leased into the solution in contact with the membrane, whereby the character of the membrane is considerably changed. Meyer and Sievers5 used an oxida- tion method to activate a cellophane membrane. -
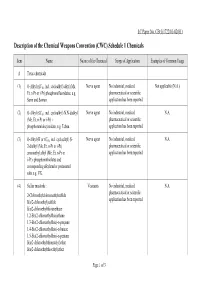
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Ep 2508506 A1
(19) & (11) EP 2 508 506 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 10.10.2012 Bulletin 2012/41 C07C 67/343 (2006.01) C07C 227/08 (2006.01) C07F 5/02 (2006.01) C07C 229/34 (2006.01) (21) Application number: 11161611.6 (22) Date of filing: 08.04.2011 (84) Designated Contracting States: (72) Inventor: The designation of the inventor has not AL AT BE BG CH CY CZ DE DK EE ES FI FR GB yet been filed GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (74) Representative: Kunic Tesovic, Barbara Designated Extension States: Lek Pharmaceuticals d.d. BA ME Sandoz Development Center Slovenia - Patents Verovskova 57 (71) Applicant: LEK Pharmaceuticals d.d. 1526 Ljubljana (SI) 1526 Ljubljana (SI) (54) Preparation of sitagliptin intermediates (57) The invention relates to the preparation of chiral compounds, in particular to the preparation of chiral compounds which may be used as intermediates for the preparation of anti-diabetic agents, preferably sitagliptin. EP 2 508 506 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 508 506 A1 Description Field of the Intention 5 [0001] The present invention relates to the preparation of chiral compounds, in particular to the preparation of chiral compounds which may be used as intermediates for the preparation of anti-diabetic agents, preferably sitagliptin. Background prior art 10 [0002] Type II diabetes mellitus (T2DM) is a global epidemic. Therefore, the research is oriented in the development of selective inhibitors of the enzyme DPP-IV as a promising new treatment for the type II diabetes.