334 Florida Entomologist 77(3) September, 1994
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
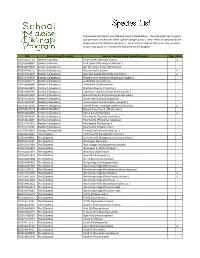
FSMTP Individual Species Lists Final VB.Xlsx
The species collected in your Malaise trap are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in your trap out of all 21 that were deployed for the program. BIN Group (scientific name) Species common name (scientific name) New Rare BOLD:AAE0114 Spiders (Araneae) Pirate spider (Mimetus notius ) ✓ BOLD:AAA8847 Spiders (Araneae) Crab spider (Misumessus oblongus ) BOLD:AAH2753 Beetles (Coleoptera) Ant‐like flower beetle (Anthicidae) BOLD:AAH0141 Beetles (Coleoptera) Ground beetle (Lebia ) ✓ BOLD:AAD4097 Beetles (Coleoptera) Bean leaf beetle (Cerotoma trifurcata ) ✓ BOLD:AAD4999 Beetles (Coleo(Coleoptera)ptera)Western corn rootworm (Diabrotica virvirgiferagifera ) BOLD:AAN6151 Beetles (Coleoptera) Leaf beetle (Longitarsus ) BOLD:ABA9949 Beetles (Coleoptera) Leaf beetle (Chaetocnema ) BOLD:AAU6970 Beetles (Coleoptera) Checkered beetle (Enoclerus ) BOLD:AAB5640 Beetles (Coleoptera) Halloween lady beetle (Harmonia axyridis ) BOLD:AAD7604 Beetles (Coleoptera) Spotted lady beetle (Coleomegilla maculata ) BOLD:AAH0130 Beetles (Coleoptera) Clover weevil (Sitona hispidulus ) BOLD:ABA6367 Beetles (Coleoptera) Plum curculio (Conotrachelus nenuphar ) BOLD:ACD4236 Beetles (Coleoptera) Minute brown scavenger beetle (Corticarina ) ✓ BOLDBOLD:AAH AAH01340134 BeetlesBeetles (Coleoptera)(Coleoptera) ShiningShining floflower er beetlebeetle (Phalacridae)(Phalacridae) BOLD:AAN6202 Beetles -

Cabbage Looper, Trichoplusia Ni (Hübner) (Insecta: Lepidoptera: Noctuidae)1 John L
EENY-116 Cabbage Looper, Trichoplusia ni (Hübner) (Insecta: Lepidoptera: Noctuidae)1 John L. Capinera2 Distribution stages. In Florida, continuous activity and reproduction occur only south of Orlando. The remainder of Florida and The cabbage looper is found throughout much of the world the portion of Georgia south of Byron, as well as southeast where crucifers are cultivated, and during the summer South Carolina, have intermittent adult activity during the months can be found throughout most of the USA. How- winter months, depending on weather.All points north of ever, overwintering in the US apparently occurs only in the this have no winter activity. southernmost states. It is somewhat erratic in occurrence, typically very abundant one year, and then scarce for two Egg to three years. This is likely due to the residual effects of Cabbage looper eggs are hemispherical in shape, with a nuclear polyhedrosis virus, which is quite lethal to this the flat side affixed to foliage. They are deposited singly insect. The cabbage looper is highly dispersive, and adults on either the upper or lower surface of the leaf, although have sometimes been found at high altitudes and far from clusters of six to seven eggs are not uncommon. The eggs shore. Flight ranges of approximately 200 km have been are yellowish white or greenish in color, bear longitudinal estimated. ridges, and measure about 0.6 mm in diameter and 0.4 mm in height. Eggs hatch in about two, three, and five days at Description and Life Cycle 32, 27, and 20°C, respectively, but require nearly 10 days at The number of generations completed per year varies from 15°C (Jackson et al. -

The Zebra Longwing Butterfly
A Special Visitor to West Central Louisiana and Almost Eden: The Zebra Longwing Butterfly For the first time this past fall we were graced with the presence of Zebra Longwing Butterflies, Heliconius charithonia. These big bold beautiful butterflies are ‘sometimes visitors’ to the warm southern and more tropical regions of Louisiana and are permanent residents of Florida (their state butterfly) and the Lower Rio Grande Valley of south Texas. They migrate north to other portions of the US and have been reported from as far north as Illinois, Colorado, Virginia, and even New York (you think they could have shown up a little sooner, lol). They were here from October until mid-December of 2016. This is one of the easiest butterflies to recognize and of the first a budding butterfly enthusiast like myself to have learned and one I had always yearned to see. Their long delicate wings and seemingly non-stop fluttering give rise to a fairy-like appearance. The long dark wings with broad bold wide buttery yellow stripes are actually warning signs to predators that this butterfly is poisonous due to the fact that the caterpillars consume Passionvines which have poisonous components that the insects take up as a defense against predation in the same way the Monarch butterfly does. The Zebra Longwing is reported to have spatial memory and will return to the same plants each day in search of nectar. And like the Julia, they also have an especially strong affinity for the flowers of Lantana but are not against visiting other similarly nectar rich species such as Porterweed, Mexican Firebush, and Pentas. -

Cervecera. Areas Sembradas Y Graduados, Facultades Y Empresas, Producción En Los Últimos Dos Decenios
ACADEMIA NACIONAL DE AGRONOMIA Y VETERINARIA ANALES TOMO XLVI 1992 BUENOS AIRES REPUBLICA ARGENTINA ACADEMIA NACIONAL DE AGRONOMIA Y VETERINARIA Fundada el 16 de Octubre de 1909 Avenida Alvear 1711, 2Q P., Tel. /Fax: 812-4168 C.P. 1014, Buenos Aires, República Argentina MESA DIRECTIVA Presidente Dr. Norberto Ras Vicepresidente Ing. Agr. Diego J. Ibarbia Secretario General Dr. Alberto E. Cano Secretario de Actas Ing. Agr. Manuel V. Fernández Valiela Tesorero Dr. Jorge Borsella Protesorero ACADEMICOS DE NUMERO Dr. Héctor G. Aramburu A rq. Pablo Hary Ing. Agr. Héctor O. Arriaga Ing. Agr.Juan H. Hunziker Ing. Agr. Wilfredo H. Barrett Ing. Agr.Diego J. Ibarbia Dr. Jorge Borsella Ing. Agr.Walter F. Kugler Dr. Raúl Buide Dr. Alfredo Manzullo Ing. Agr. Juan J. Burgos Ing. Agr.Angel Marzocca Dr. Angel L. Cabrera Ing. Agr.Edgardo R. Montaldi Dr. Alberto E. Cano Dr. Emilio G. Morini Dr. Bernardo J. Carrillo Dr. Rodolfo M. Perotti Dr. Pedro Cattáneo +Ing. Agr.Arturo E. Ragonese Ing. Agr. Milán J. Dimitri Dr Norberto Ras t Ing. Agr. Ewald Favret Ing. Agr.Manfredo A.L. Reichart Ing. Agr. Manuel V. Fernández Valiela Ing. Agr.Norberto A.R. Reichart Dr. Guillermo G. Gallo Ing. Agr.Luis De Santis Dr. Enrique García Mata Ing. Agr.Alberto Soriano Ing. Agr. Rafael García Mata Dr. Ezequiel C. Tagle Ing. Agr. Roberto E. Halbinger Ing. Agr.Esteban A. Takacs ACADEMICOS HONORARIOS Ing. Agr. Dr. Norman E. Borlaug (Estados Unidos) Ing. Agr. Dr. Theodore Schultz (Estados Unidos) ACADEMICOS CORRESPONDIENTES Ing. Agr. Ruy Barbosa Ing. Agr. Jorge A. Mariotti (Chile) (Argentina) Dr. -

Chalcid Forum Chalcid Forum
ChalcidChalcid ForumForum A Forum to Promote Communication Among Chalcid Workers Volume 23. February 2001 Edited by: Michael E. Schauff, E. E. Grissell, Tami Carlow, & Michael Gates Systematic Entomology Lab., USDA, c/o National Museum of Natural History Washington, D.C. 20560-0168 http://www.sel.barc.usda.gov (see Research and Documents) minutes as she paced up and down B. sarothroides stems Editor's Notes (both living and partially dead) antennating as she pro- gressed. Every 20-30 seconds, she would briefly pause to Welcome to the 23rd edition of Chalcid Forum. raise then lower her body, the chalcidoid analog of a push- This issue's masthead is Perissocentrus striatululus up. Upon approaching the branch tips, 1-2 resident males would approach and hover in the vicinity of the female. created by Natalia Florenskaya. This issue is also Unfortunately, no pre-copulatory or copulatory behaviors available on the Systematic Ent. Lab. web site at: were observed. Naturally, the female wound up leaving http://www.sel.barc.usda.gov. We also now have with me. available all the past issues of Chalcid Forum avail- The second behavior observed took place at Harshaw able as PDF documents. Check it out!! Creek, ~7 miles southeast of Patagonia in 1999. Jeremiah George (a lepidopterist, but don't hold that against him) and I pulled off in our favorite camping site near the Research News intersection of FR 139 and FR 58 and began sweeping. I knew that this area was productive for the large and Michael W. Gates brilliant green-blue O. tolteca, a parasitoid of Pheidole vasleti Wheeler (Formicidae) brood. -

Introduced and Native Leafrollers (Lepidoptera: Tortricidae) on Berry Crops in the Lower Fraser Valley, B
INTRODUCED AND NATIVE LIZAFROLLERS (LEPIDOPTERA : TORTRICIDAE) ON BERRY CROPS IN THE LOWER FRASER VALLEY, B .C. David Roy ~illespie B .Sc,, Simon Fraser University, 1975 M~SC.,Simon Fraser university, 1979 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMFNTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in the Department of Biological Sciences @ David Roy Gillespie 1981 SIMON FRASER UNIVERSITY December 1981 All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without permission of the author. Approval Name : David R. Gillespie Degree: Doctor of Philosophy Title of Thesis: Introduced and native leafrollers (Lepidoptera: Tortricidae) on berry crops in the Lower Fraser Valley, B. C. Examining Committee Chairman: Dr. Robert C. Brooke Dr. B. P. B'ehne, Senior Supervisor Dr. J. H. Borden ~f.J. Raine Dr. P. Belton, Public Examiner ~r:stanlei G.-- oyt, ~ntomolo~ist,Tree Fruit Research, Hentre, Washington State University, Washington, U.S.A. External Examiner Date approved: 9L- &. /%Y ----PART IAt- COPYR l GHT L I CENSE I hereby grant to Simon Fraser University the right to lend my thesis, project or extended essay (the title sf which is shown below) to users of the Simon Fraser University Library, and to make partial or single copies only for such users or in response to a reqbest from the library of any other university, or other educational institution, on its own behalf or for one of its users. I further agree that permission for multiple copying of this work for scholarly purposes may be granted by me or the Dean of Graduate Studies. -

Its Natural Enemies, and Th
The Effects of Strawberry Cropping Practices on the Strawberry Tortricid (Lepidoptera: Tortricidae), Its Natural Enemies, and the Presence of Nematodes Author(s): Lene Sigsgaard, Cyril Naulin, Solveig Haukeland, Kristian Kristensen, Annie Enkegaard, Nauja Lisa Jensen, Jørgen Eilenberg Source: Journal of Insect Science, 14(122):1-18. Published By: Entomological Society of America URL: http://www.bioone.org/doi/full/10.1673/031.014.122 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Insect Science: Vol. 14 | Article 122 Sigsgaard et al. The effects of strawberry cropping practices on the strawberry tortricid (Lepidoptera: Tortricidae), its natural -

Spite: Evolution Finally Gets Nasty
Spite: Evolution Finally Gets Nasty Altruism's "neglected ugly sister" comes to the party | By Stuart Blackman Courtesy Michael R. Strand The body of a caterpillar is the site of both a great feast and a gruesome familial struggle. But unlike even the most dys-functional holiday dinners, this fight for food erupts into bloodbath, with sisters killing sisters and brothers alike. The slaughter, as damaging to killer as to killed, exemplifies an ugly facet of evolution – the role of spite. Partaking in this grisly feast are the larvae of a parasitoid wasp, Copidosoma floridanum. Like other wasps, Copidosoma are haplodiploid: Fertilized eggs produce females; unfertilized eggs become males. These wasps are also polyembryonic: Eggs split to produce many clonal embryos. A single host may contain multiple eggs from multiple females, resulting in a hodgepodge of genetic relationships. The violence erupts when a proportion of the larvae (mostly females) develop into sterile soldiers armed with large mandibles, whose sole purpose is to seek out and kill less-related larvae. A LARVAL FEAST: Some have described spiteful behavior in Copidosoma floridanum. These wasps lay their eggs in the egg of moth, Trichoplusia ni. The host larva as a fourth instar is shown at top. Copidosoma fight for resources as they consume the caterpillar. Some differentiate into sterile soldiers foregoing reproduction to preferentially kill less-related larvae. In the so-called mummy (middle) the outlines of reproductive larvae are visible as they pupate. Eventually, they emerge as adults (bottom). Soldier larvae, in contrast, die inside the mummy. "They're more nasty to sisters than they are to clonal sisters. -

Precocious Larvae in the Polyembryonie Parasitoid Copidosoma Sosares (Hymenoptera: Encyrtidae)
Precocious larvae in the polyembryonie parasitoid Copidosoma sosares (Hymenoptera: Encyrtidae) Ian C.W. Hardy HARDY, I.C.W., 1996. PRECOCIOUS LARVAE IN THE POLYEMBRYONIC PARASITOID COPIDOSOMA SO¬ SARES (HYMENOPTERA: ENCYRTIDAE). - ENT. BER., AMST. 56 (5): 88-92. Abstract: In polyembryonie Hymenoptera, many larvae develop clonally from a single egg. Some species exhibit with- in-clone dimorphism: most larvae eventually develop into adult parasitoids, but a minority of precociously developing larvae differ in form and die before pupation. Precocious larvae in some (and possibly all) species defend their genetical¬ ly identical siblings from competition by other parasitoids. In this paper I document, for the first time, the occurrence and prevalence of precocious larvae in Copidosoma sosares (Hymenoptera: Encyrtidae) and discuss their possible function. Institute of Evolutionary and Ecological Sciences, University of Leiden, P.O. Box 9516, 2300 RA Leiden, The Netherlands. Introduction competing endoparasitic species (Cruz, 1981, 1986a; Grbic & Strand, 1991) offering a com¬ Polyembryony, defined as the production of pelling explanation for the observed success multiple embryos from a single egg, has evol¬ of polyembryonie species faced with inter¬ ved in four families of parasitoid Hymeno¬ specific competition (Browning & Oatman, ptera: Braconidae, Platygasteridae, Dryinidae 1984; Cruz, 1986a; Strand et al., 1990). Fur¬ and the Encyrtidae (Ivanova-Kasas, 1972). thermore, precocious larvae developing from Polyembryony is the most extreme in the cop- female C. floridanum eggs attack their male idosomatine encyrtids where up to 3000 larvae siblings when male and female eggs are laid in can be produced from just one egg (Ode & the same host (Grbic et al., 1992). Intersexual Strand, 1995). -

Hawai'i Landscape Plant Pest Guide: Chewing Insects
Insect Pests February 2015 IP-37 Hawai‘i Landscape Plant Pest Guide: Chewing Insects Arnold Hara & Ruth Niino-DuPonte Department of Plant & Environmental Protection Sciences Banana Moth Caterpillar (Opogona sacchari) Identification and Damage • Mobile and voracious, banana moth larvae feed on detritus and decaying plant material then move on to adjacent healthy tissue, boring into the stem and feeding on the cortex and pith. • Caterpillars avoid light within the holes they bore in plant tissue but can be detected by the accumulation of frass and debris bound with silky Banana moth eggs. (Photo credit: Robert Hollingsworth, secretions. USDA-ARS-PBARC.) • Host plants include tropical crops such as banana, eggplant, pineapple, bamboo, maize, peppers, sugarcane, and coffee, and ornamentals, including Alpinia, Begonia, Bougainvillea, bromeliads, cactus, Dracaena, Dieffenbachia, Ficus, Helicona, palms, Strelitzia, and ti. • Pupation occurs in plant tissue or in soil beneath host plant. As the pupa matures, it works itself partially out to allow adult to emerge. Larval stage. (Photo credit: Walter Nagamine, HDOA.) • Adult moths are nocturnal. At rest, their long antennae point forward. What to Do • Remove decaying and dead plant tissue. • Insecticides are not effective against the caterpillar once it bores into the plant stem. Contact insecticides such as pyrethroids may be used as a preventative treatment, controlling caterpillars prior to their entry into plant tissue. Adult moth and pupal case. More Information • Sugano, J., and R.F.L. Mau. 2001. Crop Profile for Bananas in Hawai‘i. University of Hawai‘i IPM. • EPPO Data Sheets on Quarantine Pests: Opogona sacchari. http://www. eppo.org/QUARANTINE/insects/Opogona_sacchari/OPOGSC_ds.pdf Adult moths Published by the College of Tropical Agriculture and Human Resources (CTAHR) and issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in co- operation with the U.S. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Hymenoptera für 1904. Bearbeitet von Dr. Robert Lucas in Rixdorf bei Berlin. A. I'iibliliationen (Autoren alphabetisch). Aaron, S. Frank (I). 1901, The Life History of an Insect Parasite. Scient. Amer. vol. 84. p. 394, 3 figg. Rhogas harrisinae. — {%). The Parasite of the Oak Primer. Scient. Amer. vol. 90. p. 179 Acloque, A. (1). 1896. Les entomocecidies. Le Cosmos Ann. 45. vol. 1. p. 199—204, 8 figs. — {%). Les guepes entomophages. op. cit. Ann. 45. vol. 1. p. 137 —141, Ifig. — (3). 1900. Les hotes des Fourmilieres. op. cit. N. S. T. 43. p. 393 —397. 5figgs. — (-4). 1900. Quelques ennemies des Pucerons. op. cit. N. S. T. 43. p. 740—744, 9 figs. — (5). 1904. Sphex et Ichneumons, op. cit. Ann. 50. p. 361—363, 1 fig. — (6). Les Hymenopteres a larves entomophages. Op. cit. N. S. T. 51. p. 232—236, 6 figs. Adlerz, dJottfried (I). Utvecklingen af ett Polistes-samhälle. Entom. Tidskr. Arg. 25. p. 97—106. — (2). Om cellbvggnad och tjufbin hos Trachusa serratulae Panz. t. c. p. 121—129. — (3). La proie de Methoca ichneumonides Latr. Ark. Zool. Bd. 1. p. 255—258. Alfken, J. D. (1). Beitrag zur SynonjTnie der Apiden. Zeitschrift für System. Hym. u. Dipt. 4. Jahrg. p. 1 —3. Halictus frey-gessneri nom. nov. für H. subfasciatus Nyl. non Imh. — (3). Über die von Brülle aufgestellten griechischen Andrena- Arten. t. c. p. 289—295. A. fimbriata var. paganettii n. Arch. f. Niiturgeäoli. 71. Jahrg. 1905. Bd. II. H. 2. 23 © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at 362 Dl". -

Curriculum Vitae Name
CURRICULUM VITAE NAME Paul Ode ADDRESS PHONE Bioagricultural Sciences and Pest Management (970) 491-4127 College of Agricultural Sciences Plant Sciences Campus Delivery 1177 EDUCATION 1994 Ph D, Univ Wisconsin - Madison 1990 MS, Univ Wisconsin - Madison 1986 BA, Earlham College ACADEMIC POSITIONS 2017-2018 - Associate Professor (College of Agricultural Sciences) 2016-2017 - Associate Professor (College of Agricultural Sciences) 2015-2016 - Associate Professor (College of Agricultural Sciences) 2014-2015 (College of Agricultural Sciences) 2013-2014 (College of Agricultural Sciences) 2012-2013 (College of Agricultural Sciences) 2011-2012 (College of Agricultural Sciences) 2010-2011 (College of Agricultural Sciences) 2009-2010 (College of Agricultural Sciences) OTHER POSITIONS July 1, 2011 - Present Associate Professor, Colorado State University. August 16, 2008 - June 30, 2011 Assistant Professor, Colorado State University. November 1, 2002 - August 15, 2008 Assistant Professor, North Dakota State University. 1999 - 2002 Entomologist (GS-12), USDA-ARS, Beneficial Insects Introduction Laboratory. 1998 - 1999 Postdoctoral Research Associate, Texas A&M University. 1997 - 1998 NSF-NATO Postdoctoral Fellow, Leiden University (The Netherlands). 1996 - 1997 Maytag Postdoctoral Fellow, Arizona State University. 1994 - 1996 Postdoctoral Fellow - USDA Postdoctoral Area Award, University of California - Davis. PUBLISHED WORKS Books Ode, P. J. (1994). Female-biased sex allocation in the outcrossing parasitic wasp, Bracon hebetor Say (Hymenoptera: Braconidae).: University of Wisconsin–Madison., Not Peer Reviewed/Refereed Refereed Journal Articles Brood-mate avoidance in the parasitic wasp Bracon hebetor Say. Animimal Behavior, 49, 1239-1248., Peer Reviewed/Refereed Chromosome number of the polyembryonic parasitoid Copidosoma floridanum (Ashmead) (Hymenoptera: Encyrtidae). Annals of the Entomological Society of America, 83, 834-837., Peer Reviewed/Refereed Competition and brood reduction.