W127 Common Beneficial Arthropods Found in Field Crops
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Anisodactylus Binotatus Fabr., a Carabid Beetle New to New Zealand, and a Review of the Exotic Carabid Fauna
Pacific Insects 5 (4) : 837-847 December 30, 1963 ANISODACTYLUS BINOTATUS FABR., A CARABID BEETLE NEW TO NEW ZEALAND, AND A REVIEW OF THE EXOTIC CARABID FAUNA By R. L. C. Pilgrim DEPT, OF ZOOLOGY, UNIVERSITY OF CANTERBURY, NEW ZEALAND Abstract: Anisodactylus binotatus Fabr. 1787 (Col.: Carabidae), an introduced species now established in Canterbury (South Island), New Zealand, is reported for the first time. The literature respecting other carabids sometimes recorded as introduced is reviewed; Ago- nochila binotata (White, 1846), Agonum submetallicum (White, 1846), Hypharpax australasiae (Dejean, 1829) and Pentagonica vittipennis Chaudoir, 1877 are shown to be better considered as endemic to the Australia - New Zealand area. Other species are classed as either native to New Zealand, clearly introduced though not all established, or of doubtful occurrence in New Zealand. Introduction: The Carabidae of New Zealand are predominantly endemic species, but a small number of exotic species has been recorded. This paper reports a further introduc tion to the carabid fauna of this country and concludes with a survey of recorded exotic Carabidae in New Zealand. Specimens of the newly-recorded species were collected in domestic gardens in Christ church, and were included in a collection sent for identification to Dr. E. B. Britton, British Museum (Nat. Hist.), who kindly drew the writer's attention to the fact that they were so far unreported from New Zealand. Description of adult (from New Zealand specimens) Fig. 1. Anisodactylus binotatus Fabricius, 1787 Color: Head, pronotum, elytra and femora black; tibiae and tarsi light brown to red- black ; palps and antennal segments 1-2 brown, remainder of antennae black; leg spines red-brown; head with small red spot on frons between eyes. -
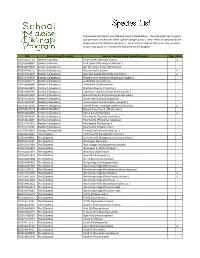
FSMTP Individual Species Lists Final VB.Xlsx
The species collected in your Malaise trap are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in your trap out of all 21 that were deployed for the program. BIN Group (scientific name) Species common name (scientific name) New Rare BOLD:AAE0114 Spiders (Araneae) Pirate spider (Mimetus notius ) ✓ BOLD:AAA8847 Spiders (Araneae) Crab spider (Misumessus oblongus ) BOLD:AAH2753 Beetles (Coleoptera) Ant‐like flower beetle (Anthicidae) BOLD:AAH0141 Beetles (Coleoptera) Ground beetle (Lebia ) ✓ BOLD:AAD4097 Beetles (Coleoptera) Bean leaf beetle (Cerotoma trifurcata ) ✓ BOLD:AAD4999 Beetles (Coleo(Coleoptera)ptera)Western corn rootworm (Diabrotica virvirgiferagifera ) BOLD:AAN6151 Beetles (Coleoptera) Leaf beetle (Longitarsus ) BOLD:ABA9949 Beetles (Coleoptera) Leaf beetle (Chaetocnema ) BOLD:AAU6970 Beetles (Coleoptera) Checkered beetle (Enoclerus ) BOLD:AAB5640 Beetles (Coleoptera) Halloween lady beetle (Harmonia axyridis ) BOLD:AAD7604 Beetles (Coleoptera) Spotted lady beetle (Coleomegilla maculata ) BOLD:AAH0130 Beetles (Coleoptera) Clover weevil (Sitona hispidulus ) BOLD:ABA6367 Beetles (Coleoptera) Plum curculio (Conotrachelus nenuphar ) BOLD:ACD4236 Beetles (Coleoptera) Minute brown scavenger beetle (Corticarina ) ✓ BOLDBOLD:AAH AAH01340134 BeetlesBeetles (Coleoptera)(Coleoptera) ShiningShining floflower er beetlebeetle (Phalacridae)(Phalacridae) BOLD:AAN6202 Beetles -

Research Article Ecological Observations of Native Geocoris Pallens and G
Hindawi Publishing Corporation Psyche Volume 2013, Article ID 465108, 11 pages http://dx.doi.org/10.1155/2013/465108 Research Article Ecological Observations of Native Geocoris pallens and G. punctipes Populations in the Great Basin Desert of Southwestern Utah Meredith C. Schuman, Danny Kessler, and Ian T. Baldwin Department of Molecular Ecology, Max Planck Institute for Chemical Ecology, Hans-Knoll-Straße¨ 8, 07745 Jena, Germany Correspondence should be addressed to Ian T. Baldwin; [email protected] Received 5 November 2012; Accepted 16 April 2013 Academic Editor: David G. James Copyright © 2013 Meredith C. Schuman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Big-eyed bugs (Geocoris spp. Fallen,´ Hemiptera: Lygaeidae) are ubiquitous, omnivorous insect predators whose plant feeding behavior raises the question of whether they benefit or harm plants. However, several studies have investigated both the potential of Geocoris spp. to serve as biological control agents in agriculture and their importance as agents of plant indirect defense in nature. These studies have demonstrated that Geocoris spp. effectively reduce herbivore populations and increase plant yield. Previous work has also indicated that Geocoris spp. respond to visual and olfactory cues when foraging and choosing their prey and that associative learning of prey and plant cues informs their foraging strategies. For these reasons, Geocoris spp. have become models for the study of tritrophic plant-herbivore-predator interactions. Here, we present detailed images and ecological observations of G. pallens Stal˚ and G. -

A Comparative Study of Two Seed Bugs, Geocoris
A COMPARATIVE STUDY OF TWO SEED BUGS, GEOCORIS BULLATUS (SAY) AND G. DISCOPTERUS STAL (HEMIPTERA: LYGAEIDAE) IN THE YUKON. By JENNIFER J. ROBINSON B.Sc. Trent University, 1980 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES DEPARTMENT OF ZOOLOGY We accept this thesis as conforming te trie required standard June, 1985 (c) Jennifer J. Robinson, 1985 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of The University of British Columbia 1956 Main Mall Vancouver, Canada V6T 1Y3 )E-6 C3/81) Abstract Geocoris bullatus (Say 1831), (Henriptera: Lygaeidae) has been collected and studied across North America but the present work is the o first detailed study of western North American CL discopterus Stal 1874. In fact, it has been claimed that 6^. discopterus is solely a species of the east. As the two species are taxonomically difficult to separate, when they were apparently discovered together at several localities in the southwestern Yukon, a detailed investigation of their systematics and distribution seemed necessary. Species status of Yukon Q. bullatus and iG. -

Cabbage Looper, Trichoplusia Ni (Hübner) (Insecta: Lepidoptera: Noctuidae)1 John L
EENY-116 Cabbage Looper, Trichoplusia ni (Hübner) (Insecta: Lepidoptera: Noctuidae)1 John L. Capinera2 Distribution stages. In Florida, continuous activity and reproduction occur only south of Orlando. The remainder of Florida and The cabbage looper is found throughout much of the world the portion of Georgia south of Byron, as well as southeast where crucifers are cultivated, and during the summer South Carolina, have intermittent adult activity during the months can be found throughout most of the USA. How- winter months, depending on weather.All points north of ever, overwintering in the US apparently occurs only in the this have no winter activity. southernmost states. It is somewhat erratic in occurrence, typically very abundant one year, and then scarce for two Egg to three years. This is likely due to the residual effects of Cabbage looper eggs are hemispherical in shape, with a nuclear polyhedrosis virus, which is quite lethal to this the flat side affixed to foliage. They are deposited singly insect. The cabbage looper is highly dispersive, and adults on either the upper or lower surface of the leaf, although have sometimes been found at high altitudes and far from clusters of six to seven eggs are not uncommon. The eggs shore. Flight ranges of approximately 200 km have been are yellowish white or greenish in color, bear longitudinal estimated. ridges, and measure about 0.6 mm in diameter and 0.4 mm in height. Eggs hatch in about two, three, and five days at Description and Life Cycle 32, 27, and 20°C, respectively, but require nearly 10 days at The number of generations completed per year varies from 15°C (Jackson et al. -

Hymenoptera: Braconidae), Parasitoids of Gramineous Stemborers in Africa
Eur. J. Entomol. 107: 169–176, 2010 http://www.eje.cz/scripts/viewabstract.php?abstract=1524 ISSN 1210-5759 (print), 1802-8829 (online) Host recognition and acceptance behaviour in Cotesia sesamiae and C. flavipes (Hymenoptera: Braconidae), parasitoids of gramineous stemborers in Africa MESHACK OBONYO1, 2, FRITZ SCHULTHESS3, BRUNO LE RU 2, JOHNNIE VAN DEN BERG1 and PAUL-ANDRÉ CALATAYUD2* 1School of Environmental Science and Development, North-West University, Potchefstroom, 2520, South Africa 2Institut de Recherche pour le Développement (IRD), UR 072, c/o International Centre of Insect Physiology and Ecology ( ICIPE), Noctuid Stemborer Biodiversity (NSBB) Project, PO Box 30772-00100, Nairobi, Kenya and Université Paris-Sud 11, 91405 Orsay, France 3ICIPE, Stemborer Biocontrol Program, PO Box 30772-00100, Nairobi, Kenya Key words. Hymenoptera, Braconidae, Cotesia sesamiae, C. flavipes, Lepidoptera, Pyralidae, Eldana saccharina, Noctuidae, Busseola fusca, Chilo partellus, parasitoids, host recognition, host acceptance, stemborers, Africa Abstract. The host recognition and acceptance behaviour of two braconid larval parasitoids (Cotesia sesamiae and C. flavipes) were studied using natural stemborer hosts (i.e., the noctuid Busseola fusca for C. sesamiae, and the crambid Chilo partellus for C. flavi- pes) and a non-host (the pyralid Eldana saccharina). A single larva was introduced into an arena together with a female parasitoid and the behaviour of the wasp recorded until it either stung the larva or for a maximum of 5 min if it did not sting the larva. There was a clear hierarchy of behavioural steps, which was similar for both parasitoid species. In the presence of suitable host larvae, after a latency period of 16–17 s, the wasp walked rapidly drumming the surface with its antennae until it located the larva. -

Investigating the Use of Buckwheat Strips for the Management of Colorado Potato Beetles in Potato Production and As an Attractan
Investigating the Use of Buckwheat Strips for the Management of Colorado Potato Beetles in Potato Production and as an Attractant of Native Pollinators for Vine Crops 1. Project Summary This project consisted of two parts. The first was to see if buckwheat strips would attract beneficial predatory insects over to adjacent planted potato rows. My hypothesis was that Colorado potato beetle larvae would have reduced numbers due to the predation by beneficial insect predators attracted by the nearby buckwheat. Compared to the control plot where no buckwheat was planted, the treatment plots showed a significant increase in larvae reduction and predation by four species of predators. The second part of the project was to see if strips of buckwheat planted next to rows of cucumbers would attract native pollinators over to the vine crops to help improve pollination (and increase marketable fruit). Compared to the control plot where no buckwheat was planted, the treatment plots had greater numbers of native bees and wasps. There also was a significant difference between the number of marketable cucumbers and those that were misshapen due to poor pollination. 2. Introduction to Topic Cover crops have great benefits for vegetable production. The cover crops can suppress weeds, reduce erosion, act as a green manure providing nutrients, and adds organic matter back to the soil. Still with all of these fine properties, cover crops still are not as commonplace on an organic farm as they should be. Many small growers have land restrictions so that every piece is needed for production. Having some tied up in cover crops means reduced sales potential in the short run. -

The Gypsy Moth and Its Natural Enemies Agriculture Information Bulletin No
THE GYPSY MOTH AND ITS NATURAL ENEMIES AGRICULTURE INFORMATION BULLETIN NO. 381 U.S. DEPARTMENT OF AGRICULTURE FOREST SERVICE i^Q^^áh nú'3^1 '/■*X. -//' ■*iS3l^ THE AUTHOR ROBERT W. CAMPBELL is principal ecologist at the North- eastern Forest Experiment Station's research unit maintained at Syracuse, N. Y., in cooperation with the State University of New York College of Environmental Science and Forestry at Syracuse University. He received his bachelor's degree in forestry from the State University of New York College of Forestry in 1953 and his master's and Ph.D. degrees in forestry from the University of Michigan in 1959 and 1961. He joined the USDA Forest Service's Northeastern Forest Experiment Station in 1961. ACKNOWLEDGMENTS My thanks to both Wayne Trimm and Robert W. Brown, whose beautiful illustrations reflect careful study of their sub- jects. I also thank the many gypsy moth watchers who have shared their observations and experiences with me. Issued February 1975 11 THE GYPSY MOTH AND ITS NATURAL ENEMIES by Robert W. Campbell CONTENTS BEHAVIOR 2 Hatch and dispersal 2 Young larvae 2 Older larvae 4 Pre-pupae and pupae 4 Adults 6 Eggs 6 MORTALITY 8 Young larvae 8 Older larvae 11 Pre-pupae 18 Pupae 18 Adults 21 Eggs 21 AGENTS THAT KILL THE SEXES DIFFERENTIALLY 22 CHANGES IN GYPSY MOTH POPULATION DENSITY 23 A FEW LAST WORDS 27 111 CAMPBELL, ROBERT W. 1974. The Gypsy Moth and its Natural Enemies. Agr. Inf. Bull. No. 381,27 p., illus. Patterns of gypsy moth behavior are described, especially those related to population density. -

Common Beneficial Insects and Their Habitat Plant Materials Technical Note
Natural Resources Conservation Service Technical Note No: TX-PM-14-02 April 2014 Common Beneficial Insects and their Habitat Plant Materials Technical Note Background: Insects are commonly viewed as pests. However, without insects earth would be a very different place. Webster’s dictionary defines pest as an animal or insect that causes problems for people, especially by damaging crops. Most insects encountered daily are not pest rather they are harmless or beneficial. Beneficial insects are any of a number of species of insects that perform vital ecological functions such as pollination, pest control, decomposition and maintenance of wildlife species. These ecological services provide an estimated annual economic value $57 billion in the United States of America. Considering the ecological and economic values, greater effort and investments should be spent in conserving these insects. NRCS-Texas Technical Note: TX-PM-14-02 Purpose: The purpose of this technical note is to provide general beneficial insect information and identify common beneficial insects and habitat that supports the occurrence of these species. Previous technical notes have addressed the value, benefit and habitat of pollinators. This technical note concentrates on insects that assist with pest management. Types of Beneficial Insects: In general, there are two kinds of pest management beneficial insects: predators and parasitoids. Predators feed directly on other insects by chewing with their mandibles or by piercing the predators and consuming the body liquids. Predators must kill and consume more than one prey to complete their development, and are free-living as immature and as adults. The action of predators is often obscure. -

Natural Pest Control Using Beneficial Insects to Control Landscape Pests
Common beneficial insects found in Beneficial insects: FS930 New Jersey Nature’s alternative to pesticides Fact sheet For a comprehensive list of our publications visit www.rce.rutgers.edu LADY BEETLE Many property owners assume that spraying pesticides is the only reliable method to kill lawn and garden Feeds on: aphids, scales, mites, pests. There is a safer and more effective way, how- and mealybugs. ever, to prevent or reduce pest damage—by promot- Natural ing natural populations of insects that feed on harm- To attract lady beetles, plant: yarrow, golden-rod, and ful pests. morning glory. Beneficial insects used in place of traditional chemi- Pest Control SPIDER cal pesticides will: Using beneficial insects to control landscape pests Feeds on: fleas, lace-bugs, and • Improve the long-term health of your plants the eggs of the Japanese beetle • Save you money by reducing your reliance on Deborah Smith-Fiola and sod webworm. costly pesticides Former, Ocean County Agricultural Agent • Help protect the environment Provide shade to draw spiders. Read this brochure to learn more about beneficial insects. GROUND BEETLE Feeds on: gypsy moths, caterpillars, weevils, and ants. Low plants, groundcovers, and camphor weed will lure ground beetles. GREEN LACEWING Feeds on: aphids, white- flies, scales, mites, and lacebugs. © 2004 by Rutgers Cooperative Research & Extension, NJAES, Rutgers, The State University of New Jersey. This beneficial insect is attracted to yarrow and wild Desktop publishing by Rutgers-Cook College Resource Center carrot. Revised: August 2003 RUTGERS COOPERATIVE RESEARCH & EXTENSION HOVER FLY N.J. AGRICULTURAL EXPERIMENT STATION RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY Feeds on: aphids, leafhoppers, NEW BRUNSWICK Distributed in cooperation with U.S. -

Arthropod Communities and Transgenic Cotton in the Western United States: Implications for Biological Control S.E
284 Naranjo and Ellsworth ___________________________________________________________________ ARTHROPOD COMMUNITIES AND TRANSGENIC COTTON IN THE WESTERN UNITED STATES: IMPLICATIONS FOR BIOLOGICAL CONTROL S.E. Naranjo1 and P.C. Ellsworth2 1U.S. Department of Agriculture, Agricultural Research Service, Phoenix, Arizona, U.S.A. 2University of Arizona, Maricopa, Arizona, U.S.A. INTRODUCTION Cotton, transgenically modified to express the insecticidal proteins of Bacillus thuringiensis (Bt), has been available commercially in the United States since 1996. Bt cotton is widely used throughout the cotton belt (Layton et al., 1999), and more than 65% of the acreage in Arizona has been planted to Bt cotton since 1997. In the low desert production areas of Arizona and California, Pectinophora gossypiella (Saunders), the pink bollworm, is the major target of Bt cotton. A number of other lepidopterous species occur in this area, but they are sporadic secondary pests of cotton whose population outbreaks are typically induced by indiscriminate use of broad-spectrum insecticides. As a result of the adoption of Bt-cotton and the coincident introduction and adoption of selective insect growth regulators for suppression of Bemisia tabaci (Gennadius), insecticide usage in Arizona cotton over the past decade as declined from a high of 12.5 applications per acre in 1995 to 1.9 in 1999 (Ellsworth and Jones, 2001). These reductions in insecticide use have broadened opportunities for all biological control approaches in cotton. Beyond concern for the maintenance of susceptibility in target pest populations there also are a number of ecological and environmental questions associated with use of transgenic crops, one of the most prominent being effects on non-target organisms. -

Baccha (Ocyptamus) Medina, B
The Syrphidae of Puerto Rico1'2 H. S. Telford3-* One cannot state with certainty when the first syrphid was collected from Puerto Rico and adjacent islands. Fabricius described a number of 1 Manuscript submitted to Editorial Board October 30, 1972. 2 Scientific paper number 3914. College of Agriculture Research Center, Washing ton State University, Pullman, Washington. Work was conducted under Project No. 0046. 3 Professor and Entomologist, Department of Entomology, Washington State University; Visiting Scientist, Department of Entomology, Agricultural Experiment Station, Mayagiiez Campus, Uío Piedras, Puerto Rico, September 1968-March 1969. This study was made possible by financial support from the Department of En tomology, Agricultural Experiment Station, Mayagiiez Campus, University of Puerto Rico, Río Piedras. I wish to thank Dr. L. P. R. F. Martorell, formerly Chairman, Department of Entomology, Agricultural Experiment Station, especially for his support and aid in all aspects of the project. Mr. Silverio Medina Gaud, Associate Entomologist, Agricultural Experiment Station, was of considerable help. He ac companied me on almost all field trips, assisted in sorting and preparing the material and made valuable field trips on his own. Dr. J. R. Vockeroth, Entomology Research Institute, Ottawa, Canada, verified many determinations and offered advice on nomenclatural problems. Others who materially aided in the loan of specimens, verified determinations or in other ways were: Dr. George Drury, U.S. Atomic Energy Commission, El Verde-Caribbean National Forest, Puerto Rico; Dr. Y. S. Sedman, Western Illinois University; Dr. L. V. Knutson, Systematic Entomology Laboratory, Agricultural Research Service, United States Department of Agriculture; Dr. P. W. Wygodzinsky, American Museum of Natural History; Dr.