Cabbage Looper, Trichoplusia Ni (Hübner) (Insecta: Lepidoptera: Noctuidae)1 John L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
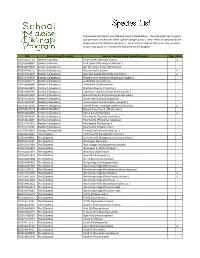
FSMTP Individual Species Lists Final VB.Xlsx
The species collected in your Malaise trap are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in your trap out of all 21 that were deployed for the program. BIN Group (scientific name) Species common name (scientific name) New Rare BOLD:AAE0114 Spiders (Araneae) Pirate spider (Mimetus notius ) ✓ BOLD:AAA8847 Spiders (Araneae) Crab spider (Misumessus oblongus ) BOLD:AAH2753 Beetles (Coleoptera) Ant‐like flower beetle (Anthicidae) BOLD:AAH0141 Beetles (Coleoptera) Ground beetle (Lebia ) ✓ BOLD:AAD4097 Beetles (Coleoptera) Bean leaf beetle (Cerotoma trifurcata ) ✓ BOLD:AAD4999 Beetles (Coleo(Coleoptera)ptera)Western corn rootworm (Diabrotica virvirgiferagifera ) BOLD:AAN6151 Beetles (Coleoptera) Leaf beetle (Longitarsus ) BOLD:ABA9949 Beetles (Coleoptera) Leaf beetle (Chaetocnema ) BOLD:AAU6970 Beetles (Coleoptera) Checkered beetle (Enoclerus ) BOLD:AAB5640 Beetles (Coleoptera) Halloween lady beetle (Harmonia axyridis ) BOLD:AAD7604 Beetles (Coleoptera) Spotted lady beetle (Coleomegilla maculata ) BOLD:AAH0130 Beetles (Coleoptera) Clover weevil (Sitona hispidulus ) BOLD:ABA6367 Beetles (Coleoptera) Plum curculio (Conotrachelus nenuphar ) BOLD:ACD4236 Beetles (Coleoptera) Minute brown scavenger beetle (Corticarina ) ✓ BOLDBOLD:AAH AAH01340134 BeetlesBeetles (Coleoptera)(Coleoptera) ShiningShining floflower er beetlebeetle (Phalacridae)(Phalacridae) BOLD:AAN6202 Beetles -

Insect Survey of Four Longleaf Pine Preserves
A SURVEY OF THE MOTHS, BUTTERFLIES, AND GRASSHOPPERS OF FOUR NATURE CONSERVANCY PRESERVES IN SOUTHEASTERN NORTH CAROLINA Stephen P. Hall and Dale F. Schweitzer November 15, 1993 ABSTRACT Moths, butterflies, and grasshoppers were surveyed within four longleaf pine preserves owned by the North Carolina Nature Conservancy during the growing season of 1991 and 1992. Over 7,000 specimens (either collected or seen in the field) were identified, representing 512 different species and 28 families. Forty-one of these we consider to be distinctive of the two fire- maintained communities principally under investigation, the longleaf pine savannas and flatwoods. An additional 14 species we consider distinctive of the pocosins that occur in close association with the savannas and flatwoods. Twenty nine species appear to be rare enough to be included on the list of elements monitored by the North Carolina Natural Heritage Program (eight others in this category have been reported from one of these sites, the Green Swamp, but were not observed in this study). Two of the moths collected, Spartiniphaga carterae and Agrotis buchholzi, are currently candidates for federal listing as Threatened or Endangered species. Another species, Hemipachnobia s. subporphyrea, appears to be endemic to North Carolina and should also be considered for federal candidate status. With few exceptions, even the species that seem to be most closely associated with savannas and flatwoods show few direct defenses against fire, the primary force responsible for maintaining these communities. Instead, the majority of these insects probably survive within this region due to their ability to rapidly re-colonize recently burned areas from small, well-dispersed refugia. -

A Magyar Természettudományi Múzeum Évkönyve 79. (Budapest 1987)
ANNALES HISTORICO-NATURALES MUSEI NATIONALIS HUNGARICI Tomus 79. Budapest, 1987 p. 167-178. Taxonomic and zoogeographical studies on the subfamily Plusiinae (Lepidoptera, Noctuidae). The Palaeotropical, Oriental and Nearctic material of the Zoological Museum, Copenhagen by L. RONKAY, Budapest L. RONKAY: Taxonomic and zoogeographical studies on the subfamily Plusiinae (Lepidoptera, Noctuidae). The Palaeotropical, Oriental and Nearctic material of the Zoological Museum, Copen hagen. — Annls hist.-nat. Mus. natn. hung. 1987 79: 167-178. Abstract — Three new genera, Anaplusia gen. n., Extremoplusia gen. n. and Scriptoplusia gen. n. and one new species, Scriptoplusia noona sp. n. are described and an annotated list of 50 species from N America, Africa and the Oriental Region is given. With 26 figures and 1 photoplate. In 1986Ihadtthe opportunity to study the Palaeotropical, Nearctic and Indo-Australian Plusiinae material of the Zoological Museum of Copenhagen. During the course of this work I could study in details some species which had not been relagated to any described genera. These studies, based on the external and genitalic morphology including the characteristics of the vesica, have shown the necessity to erect three new genera for these taxa. — The whole material contains specimens of 50 species, one of them is new for science and there are several previously unknown distribution records of the species. I would like to express my thanks to Dr. Ole Karsholt (Zool. Mus., Copenhagen) for his exten sive help in this work and also to Dr. L. Gozmány (Budapest) for his useful advice. 1. DESCRIPriON OF THE NEW TAXA It is an interesting fact that there are some species, distributed over the Eastern-South eastern border of the Palaearctic Region to Indonesia, Australia and New Guinea, which appear to be remote from any well-known genera of the Eastern Tropical Plusiinae. -

Cabbage Looper Pest Fact Sheet 11 Dr
Bringing information and education into the communities of the Granite State Cabbage Looper Pest Fact Sheet 11 Dr. Alan T. Eaton, Extension Specialist, Entomology Introduction The cabbage looper (Trichoplusia ni) is a North American native found throughout the U.S., Canada, and Mexico. It attacks all plants of the cabbage family, as well as lettuce, spinach, beets, peas, celery, parsley, potatoes, and some flower varieties. Description The larva of the cabbage looper is a small, green caterpillar with a thin white to yellow stripe on each side of the body and two stripes down the center of the back. It has three pairs of Adult cabbage looper. Credit: Whitney Cranshaw, legs near the head and three pairs of club-shaped legs (prolegs) Colorado State University, Bugwood.org. on the abdomen. The area between these legs humps into a loop during movement, giving the insect its name. The adults The cabbage looper does not overwinter are about 1" long and gray-brown in color with a 1½" wing outdoors in New Hampshire. span. The middle of the front of each wing has a silvery spot that resembles a figure 8. Life Cycle The cabbage looper does not overwinter outdoors in New Hampshire. It flies north from the south as early as mid-July, but often not until mid to late August. This accounts for the overlapping of stages in New Hampshire. Once the adult reaches New Hampshire, it lays eggs singly on the upper surfaces of the host plant. Each female lays 275-350 eggs. Upon hatching, the larva immediately feeds upon the host plant and completes development in 2-4 weeks. -

Twenty-Five Pests You Don't Want in Your Garden
Twenty-five Pests You Don’t Want in Your Garden Prepared by the PA IPM Program J. Kenneth Long, Jr. PA IPM Program Assistant (717) 772-5227 [email protected] Pest Pest Sheet Aphid 1 Asparagus Beetle 2 Bean Leaf Beetle 3 Cabbage Looper 4 Cabbage Maggot 5 Colorado Potato Beetle 6 Corn Earworm (Tomato Fruitworm) 7 Cutworm 8 Diamondback Moth 9 European Corn Borer 10 Flea Beetle 11 Imported Cabbageworm 12 Japanese Beetle 13 Mexican Bean Beetle 14 Northern Corn Rootworm 15 Potato Leafhopper 16 Slug 17 Spotted Cucumber Beetle (Southern Corn Rootworm) 18 Squash Bug 19 Squash Vine Borer 20 Stink Bug 21 Striped Cucumber Beetle 22 Tarnished Plant Bug 23 Tomato Hornworm 24 Wireworm 25 PA IPM Program Pest Sheet 1 Aphids Many species (Homoptera: Aphididae) (Origin: Native) Insect Description: 1 Adults: About /8” long; soft-bodied; light to dark green; may be winged or wingless. Cornicles, paired tubular structures on abdomen, are helpful in identification. Nymph: Daughters are born alive contain- ing partly formed daughters inside their bodies. (See life history below). Soybean Aphids Eggs: Laid in protected places only near the end of the growing season. Primary Host: Many vegetable crops. Life History: Females lay eggs near the end Damage: Adults and immatures suck sap from of the growing season in protected places on plants, reducing vigor and growth of plant. host plants. In spring, plump “stem Produce “honeydew” (sticky liquid) on which a mothers” emerge from these eggs, and give black fungus can grow. live birth to daughters, and theygive birth Management: Hide under leaves. -

Relative Attraction of the Cabbage Looper Moth (Trichoplusia Ni (Hübner)) to Wild-Type and Transgenic Tomato (Solanum Lycopersicum L.)
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 11-10-2017 9:00 AM Relative Attraction of the Cabbage Looper Moth (Trichoplusia ni (Hübner)) to Wild-type and Transgenic Tomato (Solanum lycopersicum L.) William J. Laur The University of Western Ontario Supervisor Dr. Ian Scott The University of Western Ontario Co-Supervisor Dr. Jeremy McNeil The University of Western Ontario Graduate Program in Biology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © William J. Laur 2017 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Entomology Commons Recommended Citation Laur, William J., "Relative Attraction of the Cabbage Looper Moth (Trichoplusia ni (Hübner)) to Wild-type and Transgenic Tomato (Solanum lycopersicum L.)" (2017). Electronic Thesis and Dissertation Repository. 5078. https://ir.lib.uwo.ca/etd/5078 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract The cabbage looper moth (CLM), Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) is an agricultural pest that has developed resistance to many frequently used insecticides, so alternative methods are required to reduce greenhouse CLM populations. Host plant volatile organic chemicals (VOCs) are used by female CLMs as cues for host location and oviposition. I hypothesized that changes in host plant VOC production, through genetic modification, could alter host location behaviour by CLMs. These changes in VOCs have potential to give rise to highly attractive transgenic trap crops. -

Insect Pest Management in Soybeans 12 by G
Chapter Insect Pest Management in Soybeans 12 by G. Lorenz, D. Johnson, G. Studebaker, C. Allen and S. Young, III he importance of insect pests in Arkansas Finally, it is important to determine what soybeans is extremely variable from year to management tactics are available and whether or year due in large part to environmental not they are economically feasible. T conditions. For example, hot, dry years favor many lepidopterous pests such as the soybean Insect Identification podworm and the beet armyworm; and when drought conditions occur, these pests usually are The three types of insect pests found in soybeans abundant. Many other lepidopterous pests, such as in Arkansas are: the velvetbean caterpillar and the soybean looper, 1. Foliage feeders, which comprise the biggest may cause problems following migrations from group of insect pests, southern areas, particularly in concurrence with winds out of the Gulf region where they are a 2. Pod feeders, which are probably the most common problem. Generally, insect pressure is detrimental to yield, and greater in the southern part of the state compared to 3. Stem, root and seedling feeders, which are northern Arkansas due to warmer temperatures and often the hardest to sample and are not detected closeness to the aforementioned migration sources. until after they have caused damage. Production practices also have an impact on the Some insects, such as the bean leaf beetle, may feed GEMENT occurrence of pest insects in soybeans. For example, on both foliage and pods but are primarily insects such as the Dectes stem borer and grape considered foliage feeders. -

Soybean Insects Loopers
W199 Soybean Insects Loopers Scott Stewart, Professor, Entomology and Plant Pathology Angela Thompson McClure, Associate Professor, Plant Sciences and Russ Patrick, Professor, Entomology and Plant Pathology Classification and Description: Two kinds of with a characteristic inch-worm, looping fashion. Both loopers often infest soybeans grown in Tennessee. The soybean and cabbage loopers can be distinguished cabbage looper from other caterpillars commonly found in soybean (Trichoplusia because they have three pairs of prolegs on the ni) and abdomen (one pair at the tip of the abdomen and two soybean looper additional pairs). Unlike the larvae of cabbage loopers, (Pseudoplusia soybean loopers often have black true legs (located includens) both behind the head) and/or black spots on the body. belong to the same family Hosts and Distribution: Both species of loopers have of insects a relatively wide host range and may be found on a (Lepidoptera: number of wild hosts, vegetables and other field crops Noctuidae) and such as cotton. Cabbage loopers are native to most are difficult to distinguish from each other. The moths of North America. Soybean loopers are subtropical of both species range from brown to black with a in origin, and infestations in Tennessee result from wing span of about 1 1/3 inches. The forewings of the migration of moths from southern latitudes. both species are normally mottled, often with a gold Consequently, soybean looper infestations are more or bronze sheen and prominent silver markings near common in states bordering the Gulf Coast and during the center. Eggs are typically laid singly and are late season in Tennessee. -

Tropical Insect Chemical Ecology - Edi A
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT – Vol.VII - Tropical Insect Chemical Ecology - Edi A. Malo TROPICAL INSECT CHEMICAL ECOLOGY Edi A. Malo Departamento de Entomología Tropical, El Colegio de la Frontera Sur, Carretera Antiguo Aeropuerto Km. 2.5, Tapachula, Chiapas, C.P. 30700. México. Keywords: Insects, Semiochemicals, Pheromones, Kairomones, Monitoring, Mass Trapping, Mating Disrupting. Contents 1. Introduction 2. Semiochemicals 2.1. Use of Semiochemicals 3. Pheromones 3.1. Lepidoptera Pheromones 3.2. Coleoptera Pheromones 3.3. Diptera Pheromones 3.4. Pheromones of Insects of Medical Importance 4. Kairomones 4.1. Coleoptera Kairomones 4.2. Diptera Kairomones 5. Synthesis 6. Concluding Remarks Acknowledgments Glossary Bibliography Biographical Sketch Summary In this chapter we describe the current state of tropical insect chemical ecology in Latin America with the aim of stimulating the use of this important tool for future generations of technicians and professionals workers in insect pest management. Sex pheromones of tropical insectsUNESCO that have been identified to– date EOLSS are mainly used for detection and population monitoring. Another strategy termed mating disruption, has been used in the control of the tomato pinworm, Keiferia lycopersicella, and the Guatemalan potato moth, Tecia solanivora. Research into other semiochemicals such as kairomones in tropical insects SAMPLErevealed evidence of their presence CHAPTERS in coleopterans. However, additional studies are necessary in order to confirm these laboratory results. In fruit flies, the isolation of potential attractants (kairomone) from Spondias mombin for Anastrepha obliqua was reported recently. The use of semiochemicals to control insect pests is advantageous in that it is safe for humans and the environment. The extensive use of these kinds of technologies could be very important in reducing the use of pesticides with the consequent reduction in the level of contamination caused by these products around the world. -

The Zebra Longwing Butterfly
A Special Visitor to West Central Louisiana and Almost Eden: The Zebra Longwing Butterfly For the first time this past fall we were graced with the presence of Zebra Longwing Butterflies, Heliconius charithonia. These big bold beautiful butterflies are ‘sometimes visitors’ to the warm southern and more tropical regions of Louisiana and are permanent residents of Florida (their state butterfly) and the Lower Rio Grande Valley of south Texas. They migrate north to other portions of the US and have been reported from as far north as Illinois, Colorado, Virginia, and even New York (you think they could have shown up a little sooner, lol). They were here from October until mid-December of 2016. This is one of the easiest butterflies to recognize and of the first a budding butterfly enthusiast like myself to have learned and one I had always yearned to see. Their long delicate wings and seemingly non-stop fluttering give rise to a fairy-like appearance. The long dark wings with broad bold wide buttery yellow stripes are actually warning signs to predators that this butterfly is poisonous due to the fact that the caterpillars consume Passionvines which have poisonous components that the insects take up as a defense against predation in the same way the Monarch butterfly does. The Zebra Longwing is reported to have spatial memory and will return to the same plants each day in search of nectar. And like the Julia, they also have an especially strong affinity for the flowers of Lantana but are not against visiting other similarly nectar rich species such as Porterweed, Mexican Firebush, and Pentas. -

Vegetable Insects Department of Entomology
E-99-W Vegetable Insects Department of Entomology MANAGING INSECT PESTS OF COMMERCIALLY GROWN CRUCIFERS Ricky E. Foster, Extension Entomologist The crucifers include cabbage, caulifl ower, broccoli, The following practices will reduce cabbage maggot injury. Brussels sprouts, turnips, radishes, kale, rutabaga, mustard, • Disk crop residues immediately after harvest to reduce collards, horseradish, and other crucifers. All of the crucifers overwintering populations. are subject to attack by insects. Some, such as radishes, can • Plant in well-drained soils when soil temperatures exceed usually be grown without insect damage and others, such as 50°F. cabbage, must be managed carefully to avoid serious insect • Do not plant in fi elds to which animal manure has been damage. recently applied or in which a cover crop has been plowed down within 3-4 weeks of planting. CABBAGE MAGGOTS • Use the soil insecticides diazinon, Lorsban, or Capture LFR in the seed furrow or as transplant drenches. The fi rst insect of concern on crucifers is usually the cab- bage maggot. Cabbage maggot overwinters as pupae in the FLEA BEETLES soil. The fl ies, slightly smaller than a housefl y, emerge from the soil in late April or early May and lay white eggs at the Flea beetles are almost always a pest of crucifers, es- bases of newly set plants. Emergence usually coincides with pecially early in the growing season. Flea beetles are small, the time when yellow rocket, a common weed, is in full bloom. hard-shelled insects, so named because their enlarged hind Larvae from this fi rst generation tunnel in the roots of legs allow them to jump like fl eas when disturbed. -

Cervecera. Areas Sembradas Y Graduados, Facultades Y Empresas, Producción En Los Últimos Dos Decenios
ACADEMIA NACIONAL DE AGRONOMIA Y VETERINARIA ANALES TOMO XLVI 1992 BUENOS AIRES REPUBLICA ARGENTINA ACADEMIA NACIONAL DE AGRONOMIA Y VETERINARIA Fundada el 16 de Octubre de 1909 Avenida Alvear 1711, 2Q P., Tel. /Fax: 812-4168 C.P. 1014, Buenos Aires, República Argentina MESA DIRECTIVA Presidente Dr. Norberto Ras Vicepresidente Ing. Agr. Diego J. Ibarbia Secretario General Dr. Alberto E. Cano Secretario de Actas Ing. Agr. Manuel V. Fernández Valiela Tesorero Dr. Jorge Borsella Protesorero ACADEMICOS DE NUMERO Dr. Héctor G. Aramburu A rq. Pablo Hary Ing. Agr. Héctor O. Arriaga Ing. Agr.Juan H. Hunziker Ing. Agr. Wilfredo H. Barrett Ing. Agr.Diego J. Ibarbia Dr. Jorge Borsella Ing. Agr.Walter F. Kugler Dr. Raúl Buide Dr. Alfredo Manzullo Ing. Agr. Juan J. Burgos Ing. Agr.Angel Marzocca Dr. Angel L. Cabrera Ing. Agr.Edgardo R. Montaldi Dr. Alberto E. Cano Dr. Emilio G. Morini Dr. Bernardo J. Carrillo Dr. Rodolfo M. Perotti Dr. Pedro Cattáneo +Ing. Agr.Arturo E. Ragonese Ing. Agr. Milán J. Dimitri Dr Norberto Ras t Ing. Agr. Ewald Favret Ing. Agr.Manfredo A.L. Reichart Ing. Agr. Manuel V. Fernández Valiela Ing. Agr.Norberto A.R. Reichart Dr. Guillermo G. Gallo Ing. Agr.Luis De Santis Dr. Enrique García Mata Ing. Agr.Alberto Soriano Ing. Agr. Rafael García Mata Dr. Ezequiel C. Tagle Ing. Agr. Roberto E. Halbinger Ing. Agr.Esteban A. Takacs ACADEMICOS HONORARIOS Ing. Agr. Dr. Norman E. Borlaug (Estados Unidos) Ing. Agr. Dr. Theodore Schultz (Estados Unidos) ACADEMICOS CORRESPONDIENTES Ing. Agr. Ruy Barbosa Ing. Agr. Jorge A. Mariotti (Chile) (Argentina) Dr.