Hawai'i Landscape Plant Pest Guide: Chewing Insects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Entomology Museum, University of Nebraska State 12-2009 Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii Mary Liz Jameson Wichita State University, [email protected] Darcy E. Oishi 2Hawaii Department of Agriculture, Plant Pest Control Branch, Honolulu, [email protected] Brett C. Ratcliffe University of Nebraska-Lincoln, [email protected] Grant T. McQuate USDA-ARS-PBARC, U.S. Pacific Basin Agricultural Research Center, Hilo, HI, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/entomologypapers Part of the Entomology Commons Jameson, Mary Liz; Oishi, Darcy E.; Ratcliffe, Brett C.; and McQuate, Grant T., "Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii" (2009). Papers in Entomology. 147. https://digitalcommons.unl.edu/entomologypapers/147 This Article is brought to you for free and open access by the Museum, University of Nebraska State at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. AProcddition. HawaiianAl inv AEsiventomol scA.r SAocbs. in(2009) HAwA 41:25–30ii 25 Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii Mary Liz Jameson1, Darcy E. Oishi2, Brett C. Ratcliffe3, and Grant T. McQuate4 1Wichita State University, Department of Biological Sciences, 537 Hubbard Hall, Wichita, Kansas 67260 [email protected]; 2Hawaii Department of Agriculture, Plant Pest Control Branch, 1428 South King St., Honolulu, HI 96814 [email protected]; 3University of Nebraska State Museum, Systematics Research Collections, W436 Nebraska Hall, University of Nebraska, Lincoln, Nebraska 68588 [email protected]; 4USDA-ARS-PBARC, U.S. -

New Aspects About Supella Longipalpa (Blattaria: Blattellidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Asian Pac J Trop Biomed 2016; 6(12): 1065–1075 1065 HOSTED BY Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Biomedicine journal homepage: www.elsevier.com/locate/apjtb Review article http://dx.doi.org/10.1016/j.apjtb.2016.08.017 New aspects about Supella longipalpa (Blattaria: Blattellidae) Hassan Nasirian* Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ARTICLE INFO ABSTRACT Article history: The brown-banded cockroach, Supella longipalpa (Blattaria: Blattellidae) (S. longipalpa), Received 16 Jun 2015 recently has infested the buildings and hospitals in wide areas of Iran, and this review was Received in revised form 3 Jul 2015, prepared to identify current knowledge and knowledge gaps about the brown-banded 2nd revised form 7 Jun, 3rd revised cockroach. Scientific reports and peer-reviewed papers concerning S. longipalpa and form 18 Jul 2016 relevant topics were collected and synthesized with the objective of learning more about Accepted 10 Aug 2016 health-related impacts and possible management of S. longipalpa in Iran. Like the Available online 15 Oct 2016 German cockroach, the brown-banded cockroach is a known vector for food-borne dis- eases and drug resistant bacteria, contaminated by infectious disease agents, involved in human intestinal parasites and is the intermediate host of Trichospirura leptostoma and Keywords: Moniliformis moniliformis. Because its habitat is widespread, distributed throughout Brown-banded cockroach different areas of homes and buildings, it is difficult to control. -

The Interaction of Drought and the Outbreak of Phoracantha
The interaction of drought and the outbreak of Phoracantha semipunctata (Coleoptera: Cerambycidae) on tree collapse in the Northern Jarrah (Eucalyptus marginata) forest. by Stephen Seaton (BSc Environmental Science) This thesis is presented in partial fulfilment of the requirements for the degree of Bachelor of Science (Honours) School of Biological Sciences and Biotechnology, Murdoch University, Perth, Western Australia November 2012 ii Declaration I declare that that the work contained within this thesis is an account of my own research, except where work by others published or unpublished is noted, while I was enrolled in the Bachelor of Science with Honours degree at Murdoch University, Western Australia. This work has not been previously submitted for a degree at any institution. Stephen Seaton November 2012 iii Conference Presentations Seaton, S.A.H., Matusick, G., Hardy, G. 2012. Drought induced tree collapse and the outbreak of Phoracantha semipunctata poses a risk for forest under climate change. Abstract presented at the Combined Biological Sciences Meeting (CBSM) 2012, 24th of August. University Club, University of Western Australia. Seaton, S.A.H., Matusick, G., Hardy, G. 2012. Occurrence of Eucalyptus longicorn borer (Phoracantha semipunctata) in the Northern Jarrah Forest following severe drought. To be presented at The Australian Entomological Society - 43rd AGM & Scientific Conference and Australasian Arachnological Society - 2012 Conference. 25th – 28th November. The Old Woolstore, Hobart. iv Acknowledgments I greatly appreciate the guidance, enthusiasm and encouragement and tireless support from my supervisors Dr George Matusick and Prof Giles Hardy in the Centre of Excellence for Climate Change Forests and Woodland Health. I particularly appreciate the interaction and productive discussions regarding forest ecology and entomology and proof reading the manuscript. -
Danaus Plexippus) in Milkweed Gardens and Conservation Areas
Journal of Insect Conservation https://doi.org/10.1007/s10841-018-0102-8 ORIGINAL PAPER Recruitment, survival, and parasitism of monarch butterflies (Danaus plexippus) in milkweed gardens and conservation areas Emily A. Geest1 · L. LaReesa Wolfenbarger1 · John P. McCarty1 Received: 10 July 2018 / Accepted: 24 October 2018 © The Author(s) 2018 Abstract Monarch butterflies (Danaus plexippus) are suffering from declining populations and conservationists have encouraged planting milkweed gardens in urban and suburban landscapes to help offset habitat loss across the breeding range. The effectiveness of gardens as a conservation strategy depends on their ability to attract ovipositing adults and the survival of monarch larvae in these gardens. Larvae are susceptible to a variety of predators as well as to parasitism by a tachinid fly (Lespesia archippivora) and a protozoan parasite (Ophryocystis elektroscirrha) which cause lethal or sublethal effects, yet the severity of these risks in gardens is not well understood. We compared egg abundance and larval survival in traditional conservation areas to gardens that incorporated milkweed to attract monarchs. Additionally, we collected late instar larvae and reared them in the lab to compare parasitism rates between monarch gardens and conservation areas. Both gardens and conservations sites varied widely in recruitment and survival of monarchs and there were no significant differences between the garden and conservation sites. Tachinid fly parasitism ranged from 30% of larvae from conservation sites in 2016 to 55% of larvae from gardens in 2017, but did not differ between the two categories of sites. Parasitism byO. elektroscirrha was detected in fewer than 2% of larvae. The density of milkweed had no effect on the number of monarch eggs in conservation areas or gardens in either year. -
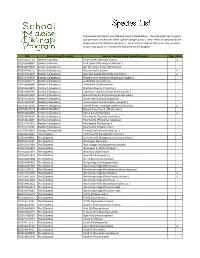
FSMTP Individual Species Lists Final VB.Xlsx
The species collected in your Malaise trap are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in your trap out of all 21 that were deployed for the program. BIN Group (scientific name) Species common name (scientific name) New Rare BOLD:AAE0114 Spiders (Araneae) Pirate spider (Mimetus notius ) ✓ BOLD:AAA8847 Spiders (Araneae) Crab spider (Misumessus oblongus ) BOLD:AAH2753 Beetles (Coleoptera) Ant‐like flower beetle (Anthicidae) BOLD:AAH0141 Beetles (Coleoptera) Ground beetle (Lebia ) ✓ BOLD:AAD4097 Beetles (Coleoptera) Bean leaf beetle (Cerotoma trifurcata ) ✓ BOLD:AAD4999 Beetles (Coleo(Coleoptera)ptera)Western corn rootworm (Diabrotica virvirgiferagifera ) BOLD:AAN6151 Beetles (Coleoptera) Leaf beetle (Longitarsus ) BOLD:ABA9949 Beetles (Coleoptera) Leaf beetle (Chaetocnema ) BOLD:AAU6970 Beetles (Coleoptera) Checkered beetle (Enoclerus ) BOLD:AAB5640 Beetles (Coleoptera) Halloween lady beetle (Harmonia axyridis ) BOLD:AAD7604 Beetles (Coleoptera) Spotted lady beetle (Coleomegilla maculata ) BOLD:AAH0130 Beetles (Coleoptera) Clover weevil (Sitona hispidulus ) BOLD:ABA6367 Beetles (Coleoptera) Plum curculio (Conotrachelus nenuphar ) BOLD:ACD4236 Beetles (Coleoptera) Minute brown scavenger beetle (Corticarina ) ✓ BOLDBOLD:AAH AAH01340134 BeetlesBeetles (Coleoptera)(Coleoptera) ShiningShining floflower er beetlebeetle (Phalacridae)(Phalacridae) BOLD:AAN6202 Beetles -

Key to the Genera of Cerambycidae of Western North America
KEY TO THE GENERA OF THE CERAMBYCIDAE OF WESTERN NORTH AMERICA Version 030120 JAMES R. LaBONTE JOSHUA B. DUNLAP DANIEL R. CLARK THOMAS E. VALENTE JOSHUA J. VLACH OREGON DEPARTMENT OF AGRICULTURE Begin key Contributions and Acknowledgements James R. LaBonte, ODA (Oregon Department of Agriculture: Design and compilation of this identification aid. Joshua B. Dunlap: Acquisition of most of the images. Daniel R. Clark: Design input and testing. Thomas E. Valente, ODA: Design input and testing. Joshua J. Vlach, ODA: Design input and testing. Thomas Shahan, Thomas Valente, Steve Valley – additional images ODA: Use of the imaging system, the entomology museum, and general support. Our appreciation to USDA Forest Service and ODA for funding this project. Introduction Begin key This identification aid is a comprehensive key to the genera of western North American Cerambycidae (roundheaded or long- horned wood borers). It also includes several genera (and species) that are either established in the region or that are targets of USDA and other exotic cerambycid surveys. Keys to commonly trapped or encountered (based on ODA’s years of wood borer surveys) indigenous species are also included. *This aid will be most reliable west of the Rocky Mountains. It may not function well with taxa found in the desert West and east of the Rockies. This aid is designed to be used by individuals with a wide range of taxonomic expertise. Images of all character states are provided. Begin key Use of This Key: I This key is designed like a traditional dichotomous key, with couplets. However, PowerPoint navigational features have been used for efficiency. -

Schools Integrated Pest Management (Ipm) for Wasps and Bees
SCHOOLS INTEGRATED PEST MANAGEMENT (IPM) FOR WASPS AND BEES *Important Note* According to the Virginia Pesticide Control Act (Section 3.1-249.53), in order to apply ANY pesticide (including Raid, Round-Up, and other over-the-counter pesticides) in public areas of ANY educational institution, the applicator must first be certified by the Virginia Department of Agriculture and Consumer Services. In other words, it is illegal for uncertified teachers, staff, administrators, or contractors to apply pesticides on school grounds. INTRODUCTION Bees and wasps experience “complete metamorphosis”. This means that they pass Wasps and bees are both beneficial and through four different life stages: egg, larva, problematic. Wasps, being predators, play pupa, and adult (see Figure 1). Individuals an important role in the control of other pest that are newly hatched are termed “larvae”. insects. Bees are essential pollinators of Larvae are blind and legless. After the plants and producers of products such as larval stage, they become pupae. The pupal honey and wax. Both vigorously defend stage is a non-feeding, immobile stage their nests and utilize stinging as their wherein the larvae transform into adults. primary defense mechanism. Although the Finally, the bees and wasps emerge from the sting of a bee or wasp is painful, it is usually pupae as fully developed adults. not life threatening. However, some individuals are extremely sensitive to the sting’s venom and may experience fatal allergic reactions. BIOLOGY AND IDENTIFICATION Bees and wasps are grouped together into the same order of insects, Hymenoptera. For the purposes of this publication the term “wasps” refers to hornets, yellowjackets, paper wasps, mud daubers, digger wasps, cicada killers, and any other wasp species that frequently are a problem around structures. -

User's Guide Project: the Impact of Non-Native Predators On
User’s Guide Project: The Impact of Non-Native Predators on Pollinators and Native Plant Reproduction in a Hawaiian Dryland Ecosystem SERDP project number: RC-2432 Principal Investigators: Christina T. Liang, USDA Forest Service Clare E. Aslan, Northern Arizona University William P. Haines, University of Hawaiʻi at Mānoa Aaron B. Shiels, USDA APHIS Contributor: Manette E. Sandor, Northern Arizona University Date: 30 October 2019 Form Approved REPORT DOCUMENTATION PAGE OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing this collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Department of Defense, Washington Headquarters Services, Directorate for Information Operations and Reports (0704-0188), 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202- 4302. Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. PLEASE DO NOT RETURN YOUR FORM TO THE ABOVE ADDRESS. 1. REPORT DATE (DD-MM-YYYY) 2. REPORT TYPE 3. DATES COVERED (From - To) 10-30-2019 User’s Guide 01-02-2014 to 10-30-2019 4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER User’s Guide. The Impact of Non-Native Predators on Pollinators and Native Plant Reproduction in a Hawaiian Dryland Ecosystem. -

Wooden and Bamboo Commodities Intended for Indoor and Outdoor Use
NAPPO Discussion Document DD 04: Wooden and Bamboo Commodities Intended for Indoor and Outdoor Use Prepared by members of the Pest Risk Analysis Panel of the North American Plant Protection Organization (NAPPO) December 2011 Contents Introduction ...........................................................................................................................3 Purpose ................................................................................................................................4 Scope ...................................................................................................................................4 1. Background ....................................................................................................................4 2. Description of the Commodity ........................................................................................6 3. Assessment of Pest Risks Associated with Wooden Articles Intended for Indoor and Outdoor Use ...................................................................................................................6 Probability of Entry of Pests into the NAPPO Region ...........................................................6 3.1 Probability of Pests Occurring in or on the Commodity at Origin ................................6 3.2 Survival during Transport .......................................................................................... 10 3.3 Probability of Pest Surviving Existing Pest Management Practices .......................... 10 3.4 Probability -

Pu'u Wa'awa'a Biological Assessment
PU‘U WA‘AWA‘A BIOLOGICAL ASSESSMENT PU‘U WA‘AWA‘A, NORTH KONA, HAWAII Prepared by: Jon G. Giffin Forestry & Wildlife Manager August 2003 STATE OF HAWAII DEPARTMENT OF LAND AND NATURAL RESOURCES DIVISION OF FORESTRY AND WILDLIFE TABLE OF CONTENTS TITLE PAGE ................................................................................................................................. i TABLE OF CONTENTS ............................................................................................................. ii GENERAL SETTING...................................................................................................................1 Introduction..........................................................................................................................1 Land Use Practices...............................................................................................................1 Geology..................................................................................................................................3 Lava Flows............................................................................................................................5 Lava Tubes ...........................................................................................................................5 Cinder Cones ........................................................................................................................7 Soils .......................................................................................................................................9 -

Cockroach Marion Copeland
Cockroach Marion Copeland Animal series Cockroach Animal Series editor: Jonathan Burt Already published Crow Boria Sax Tortoise Peter Young Ant Charlotte Sleigh Forthcoming Wolf Falcon Garry Marvin Helen Macdonald Bear Parrot Robert E. Bieder Paul Carter Horse Whale Sarah Wintle Joseph Roman Spider Rat Leslie Dick Jonathan Burt Dog Hare Susan McHugh Simon Carnell Snake Bee Drake Stutesman Claire Preston Oyster Rebecca Stott Cockroach Marion Copeland reaktion books Published by reaktion books ltd 79 Farringdon Road London ec1m 3ju, uk www.reaktionbooks.co.uk First published 2003 Copyright © Marion Copeland All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publishers. Printed and bound in Hong Kong British Library Cataloguing in Publication Data Copeland, Marion Cockroach. – (Animal) 1. Cockroaches 2. Animals and civilization I. Title 595.7’28 isbn 1 86189 192 x Contents Introduction 7 1 A Living Fossil 15 2 What’s in a Name? 44 3 Fellow Traveller 60 4 In the Mind of Man: Myth, Folklore and the Arts 79 5 Tales from the Underside 107 6 Robo-roach 130 7 The Golden Cockroach 148 Timeline 170 Appendix: ‘La Cucaracha’ 172 References 174 Bibliography 186 Associations 189 Websites 190 Acknowledgements 191 Photo Acknowledgements 193 Index 196 Two types of cockroach, from the first major work of American natural history, published in 1747. Introduction The cockroach could not have scuttled along, almost unchanged, for over three hundred million years – some two hundred and ninety-nine million before man evolved – unless it was doing something right. -

Cabbage Looper, Trichoplusia Ni (Hübner) (Insecta: Lepidoptera: Noctuidae)1 John L
EENY-116 Cabbage Looper, Trichoplusia ni (Hübner) (Insecta: Lepidoptera: Noctuidae)1 John L. Capinera2 Distribution stages. In Florida, continuous activity and reproduction occur only south of Orlando. The remainder of Florida and The cabbage looper is found throughout much of the world the portion of Georgia south of Byron, as well as southeast where crucifers are cultivated, and during the summer South Carolina, have intermittent adult activity during the months can be found throughout most of the USA. How- winter months, depending on weather.All points north of ever, overwintering in the US apparently occurs only in the this have no winter activity. southernmost states. It is somewhat erratic in occurrence, typically very abundant one year, and then scarce for two Egg to three years. This is likely due to the residual effects of Cabbage looper eggs are hemispherical in shape, with a nuclear polyhedrosis virus, which is quite lethal to this the flat side affixed to foliage. They are deposited singly insect. The cabbage looper is highly dispersive, and adults on either the upper or lower surface of the leaf, although have sometimes been found at high altitudes and far from clusters of six to seven eggs are not uncommon. The eggs shore. Flight ranges of approximately 200 km have been are yellowish white or greenish in color, bear longitudinal estimated. ridges, and measure about 0.6 mm in diameter and 0.4 mm in height. Eggs hatch in about two, three, and five days at Description and Life Cycle 32, 27, and 20°C, respectively, but require nearly 10 days at The number of generations completed per year varies from 15°C (Jackson et al.