Environmental Impact Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

The Chinese State in Ming Society
The Chinese State in Ming Society The Ming dynasty (1368–1644), a period of commercial expansion and cultural innovation, fashioned the relationship between the present-day state and society in China. In this unique collection of reworked and illustrated essays, one of the leading scholars of Chinese history re-examines this relationship and argues that, contrary to previous scholarship, which emphasized the heavy hand of the state, it was radical responses within society to changes in commercial relations and social networks that led to a stable but dynamic “constitution” during the Ming dynasty. This imaginative reconsideration of existing scholarship also includes two essays first published here and a substantial introduction, and will be fascinating reading for scholars and students interested in China’s development. Timothy Book is Principal of St. John’s College, University of British Colombia. Critical Asian Scholarship Edited by Mark Selden, Binghamton and Cornell Universities, USA The series is intended to showcase the most important individual contributions to scholarship in Asian Studies. Each of the volumes presents a leading Asian scholar addressing themes that are central to his or her most significant and lasting contribution to Asian studies. The series is committed to the rich variety of research and writing on Asia, and is not restricted to any particular discipline, theoretical approach or geographical expertise. Southeast Asia A testament George McT.Kahin Women and the Family in Chinese History Patricia Buckley Ebrey -

White Wares of Northern China
200 White Wares of Northern China Regina Krahl The white wares of northern China launched the country’s reputation as a center of porcelain. As hard, dense, and durable as their southern green counterparts, but more immediately appealing due to their sparkling, glossy, clean-looking material, white wares became the envy and aspiration of potters worldwide. Porcelain clays are naturally available in north China, and some rare examples of white wares—made of a pure, white clay, unglazed, but fired at temperatures just high enough to qualify as stonewares—have been discovered at sites of the late Shang dynasty (circa 1600–circa 1050 BCE) at Anyang in Henan province. As no continuous development, like that seen in southern stoneware, followed these early beginnings, however, they have to be considered isolated experiments, rather than origins of north China’s stoneware production. It would take another 1,600 years or so before continuous production of stonewares began in northern China and before the first white porcelains were commercialized on a regular basis. The white wares on the Belitung wreck comprised some 300 items, most of them tablewares, all made in northern China. These elegant yet utilitarian ceramics were unique to China and highly prized throughout Asia. The white wares recovered from this cargo, probably the most valuable ceramics on board, are varied in type and may represent a combination of wares from three or four different kilns. Produced mainly in Hebei and Henan provinces, they may not have been easy to come by for merchants based far away in southern port cities, even though the north was linked to the international port of Yangzhou via the Grand Canal. -

Social Monitoring Report
Social Monitoring Report Project Number: 36437 June 2012 PRC: Integrated Ecosystem and Water Resources Management in the Baiyangdian Basin Prepared by Hohai University For Hebei Baoding Baiyangdian Project Management Office This report has been submitted to ADB by the Hebei Baoding Baiyangdian Project Management Office and is made publicly available in accordance with ADB’s public communications policy (2005). It does not necessarily reflect the views of ADB . Integrated Ecosystem and Water Resources Management in the Baiyangdian Basin Project Financed by Asian Development Bank Monitoring and Evaluation Report on the Resettlement of Integrated Ecosystem and Water Resources Management in the Baiyangdian Basin Project (No. 5) National Research Center of Resettlement Hohai University, Nanjing, Jiangsu, China June, 2012 1 Persons in Charge : Shi Guoqing, Sun Yan Independent Monitoring and Shi Guoqing, Sun Yan, Hou Ronggui, : Evaluation Staff Lv Qiulong, Yuan Lin, Liu Yiying Shi Guoqing, Sun Yan, Hou Ronggui, Report Writers : Lv Qiulong, Yuan Lin, Liu Yiying Independent Monitoring and National Research Center of Resettlement : Evaluation Institute Hohai University No.1 Xikang Road Address : Nanjing, Jiangsu, China Post Code : 210098 Telephone : 0086-25-83786503 Fax : 0086-25-83718914 E-mail : [email protected] [email protected] 2 CONTENT 1 SUMMARY ................................................................................................................................................ 6 1.1 Introduction of Project ............................................................................................. -

Minimum Wage Standards in China August 11, 2020
Minimum Wage Standards in China August 11, 2020 Contents Heilongjiang ................................................................................................................................................. 3 Jilin ............................................................................................................................................................... 3 Liaoning ........................................................................................................................................................ 4 Inner Mongolia Autonomous Region ........................................................................................................... 7 Beijing......................................................................................................................................................... 10 Hebei ........................................................................................................................................................... 11 Henan .......................................................................................................................................................... 13 Shandong .................................................................................................................................................... 14 Shanxi ......................................................................................................................................................... 16 Shaanxi ...................................................................................................................................................... -

Kyle E David
UNIVERSITY OF CALIFORNIA, IRVINE Martyrs, Models, and Miscreants: The “Communist Child” in Wartime North China, 1937-1948 DISSERTATION submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in History by Kyle Ellison David Dissertation Committee Professor Jeffrey Wasserstrom, Co-chair Associate Professor Emily Baum, Co-chair Professor Susan K. Morrissey Associate Professor Anita Casavantes Bradford 2021 © 2021 Kyle Ellison David DEDICATION to Martin and Jayden whose own childhoods have been inextricably linked to those discussed herein ii TABLE OF CONTENTS LIST OF IMAGES iv ACKNOWLEDGEMENTS v CURRICULUM VITAE vi ABSTRACT OF THE DISSERTATION vii CHAPTER 1: Introduction 1 CHAPTER 2: The Birth of the Chinese Communist Child, 1922-1936 28 CHAPTER 3: Polemical Pedagogy: Educating Little Revolutionaries 67 CHAPTER 4: “Always Prepared”: The Anti-Japanese Children’s League 111 CHAPTER 5: Everyday Childhood in Japanese-Occupied North China 141 CHAPTER 6: Martyrs, Models, and Miscreants 182 CHAPTER 7: Conclusion 225 REFERENCES 237 iii LIST OF IMAGES Image 5.1 Wen Sanyu at “Heroes of the Masses” rally 200 Image 5.2 Textbook illustration of Wen Sanyu 202 iv ACKNOWLEDGEMENTS I am forever indebted to my two advisors, Dr. Jeffrey Wasserstrom and Dr. Emily Baum, for their patience, kindness, and enthusiastic support throughout the last seven years. I am grateful for the opportunity they provided upon admitting me to the doctoral program, the intellectual rigor of the training they have provided, and the promptness with which they have attended to providing feedback on my work and seemingly endless requests for letters of recommendation and other bureaucratic demands. -

I Regulations
17.5.2008EN Official Journal of the European Union L 129/1 I (Acts adopted under the EC Treaty/Euratom Treaty whose publication is obligatory) REGULATIONS COUNCIL REGULATION (EC) No 426/2008 of 14 May 2008 amending Regulation (EC) No 1212/2005 imposing a definitive anti-dumping duty on imports of certain castings originating in the People’s Republic of China THE COUNCIL OF THE EUROPEAN UNION, (2) The sampled companies were attributed the individual duty rates established during the investigation. The coop- erating non-sampled companies which were granted Having regard to the Treaty establishing the European market economy treatment (‘MET’), in accordance with Community, the provisions of Article 2(7)(c) of the basic Regulation, were attributed the 0 % dumping duty which was estab- lished for the sole sampled company which was granted MET. The cooperating non-sampled companies which Having regard to Council Regulation (EC) No 384/96 of ‘ ’ 22 December 1995 on protection against dumped imports were granted individual treatment ( IT ), in accordance from countries not members of the European Community (1) with the provisions of Article 9(5) of the basic Regu- (the ‘basic Regulation’), lation, received the weighted average duty of 28,6 % established for the sampled companies that were granted IT. A countrywide duty of 47,8 % was imposed on all other companies. Having regard to Article 1(4) of Council Regulation (EC) No 1212/2005 of 25 July 2005 imposing a definitive anti-dumping duty on imports of certain castings originating in the People’s 2 Republic of China ( ), (3) Article 1(4) of Regulation (EC) No 1212/2005 gives the possibility to Chinese exporting producers which meet the four criteria set out in that Article to be granted Having regard to the proposal submitted by the Commission the same treatment as the one mentioned in recital (2) after consulting the Advisory Committee, above for the cooperating companies not included in the sample (‘New Exporting Producer Treatment’ or ‘NEPT’). -

Canadian Social Science 70 Years of Bethune Studies in China
Canadian Social Science ISSN 1712-8056 [Print] ISSN 1923-6697[Online] Vol. 6, No. 5, 2010, pp. 94-101 www.cscanada.net www.cscanada.org 70 years of Bethune Studies in China1 70 ANNÉES D'ÉTUDES SUR BÉTHUNE EN CHINE QI Li2 Abstract: For 70 years, Bethune Studies in China is on the way from propaganda to academic research. Mao Zedong’s In memory of Norman Bethune and Zhou Erfu’s Dr. Bethune have played an important role in propagandizing the image of Bethune. The Scalpel, the Sword by Ted Allan and Sydney Gordon and The Politics of Passion -Norman Bethune's Writing and Art by Larry Hannant are especially the significant works to study Bethune. Since the beginning of 1980s some new features have been presented, such as new information, endless emerging of new works, enlarged number of researchers and the organizational trend of the study and so on. Of cause we have more work to do, which needs the cooperation and the communication between researchers in different areas, between different branches of learning or even between different countries. Key Words: Norman Bethune; Bethune Study; Canada Résumé: Depuis 70 ans en Chine, des études sur Béthune se transforment de la propagande en recherche universitaire. À la mémoire de Norman Béthune de Mao Zedong et Docteur Béthune de Zhou Erfu ont joué un rôle important dans la popularisation de l'image de Béthune. Le Scalpel et l'épée de Ted Allan et Sydney Gordon, et Politique de passion - créations et récits de Norman Béthune de Larry Hannant sont notamment des oeuvres importantes pour les études sur Bethune. -
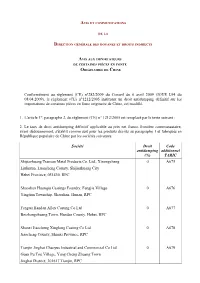
Avis Pièces En Fonte De Chine
AVIS ET COMMUNICATIONS DE LA DIRECTION GÉNÉRALE DES DOUANES ET DROITS INDIRECTS AVIS AUX IMPORTATEURS DE CERTAINES PIÈCES EN FONTE ORIGINAIRES DE CHINE Conformément au règlement (CE) n°282/2009 du Conseil du 6 avril 2009 (JOUE L94 du 08.04.2009), le règlement (CE) n°1212/2005 instituant un droit antidumping définitif sur les importations de certaines pièces en fonte originaire de Chine, est modifié. 1. L'article 1er, paragraphe 2, du règlement (CE) n° 1212/2005 est remplacé par le texte suivant : 2. Le taux de droit antidumping définitif applicable au prix net franco frontière communautaire, avant dédouanement, s'établit comme suit pour les produits décrits au paragraphe 1 et fabriqués en République populaire de Chine par les sociétés suivantes: Société Droit Code antidumping additionnel (%) TARIC Shijiazhuang Transun Metal Products Co. Ltd., Xinongcheng 0 A675 Liulintun, Luancheng County, Shijiazhuang City Hebei Province, 051430, RPC Shaoshan Huanqiu Castings Foundry, Fengjia Village 0 A676 Yingtian Township, Shaoshan, Hunan, RPC Fengtai Handan Alloy Casting Co Ltd 0 A677 Beizhangzhuang Town, Handan County, Hebei, RPC Shanxi Jiaocheng Xinglong Casting Co Ltd 0 A678 Jiaocheng County, Shanxi Province, RPC Tianjin Jinghai Chaoyue Industrial and Commercial Co Ltd 0 A679 Guan Pu Tou Village, Yang Cheng Zhuang Town Jinghai District, 301617 Tianjin, RPC Société Droit Code antidumping additionnel (%) TARIC Baoding City Maikesaier Casting Ltd. 0 A867 Xin'anli Town, Tang County Hebei, Baoding 072350, RPC Baoding Yuehai Machine Manufacturing Co., Ltd. 0 A868 No 333 Building A Tian E West Road, Baoding, Hebei, RPC Shanxi Yuansheng Casting and Forging Industrial Co. Ltd. 18,6 A680 No. -

473817 1 En Bookfrontmatter 1..67
The Metal Road of the Eastern Eurasian Steppe Jianhua Yang • Huiqiu Shao • Ling Pan The Metal Road of the Eastern Eurasian Steppe The Formation of the Xiongnu Confederation and the Silk Road 123 Jianhua Yang Huiqiu Shao Jilin University Jilin University Changchun, China Changchun, China Ling Pan Jilin University Changchun, China Translated by Haiying Pan, Zhidong Cui, Xiaopei Zhang, Wenjing Xia, Chang Liu, Licui Zhu, Li Yuan, Qing Sun, Di Yang, Rebecca O’ Sullivan. ISBN 978-981-32-9154-6 ISBN 978-981-32-9155-3 (eBook) https://doi.org/10.1007/978-981-32-9155-3 © Springer Nature Singapore Pte Ltd. 2020 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. -

Mobile Device–Based Electronic Data Capture System Used in a Clinical
JOURNAL OF MEDICAL INTERNET RESEARCH Zhang et al Original Paper Mobile Device±Based Electronic Data Capture System Used in a Clinical Randomized Controlled Trial: Advantages and Challenges Jing Zhang1*, MSc; Lei Sun1*, MSc; Yu Liu2, PhD; Hongyi Wang3, MD, PhD; Ningling Sun3, MD, PhD; Puhong Zhang1, PhD 1The George Institute for Global Health at Peking University Health Science Center, Beijing, China 2Beihang University, Beijing, China 3The People's Hospital, Peking University, Beijing, China *these authors contributed equally Corresponding Author: Puhong Zhang, PhD The George Institute for Global Health at Peking University Health Science Center Level 18, Tower B, Horizon Tower, No. 6 Zhichun Rd Haidian District | Beijing, 100088 P.R. China Beijing, China Phone: 86 1082800577 ext 512 Fax: 86 1082800177 Email: [email protected] Abstract Background: Electronic data capture (EDC) systems have been widely used in clinical research, but mobile device±based electronic data capture (mEDC) system has not been well evaluated. Objective: The aim of our study was to evaluate the feasibility, advantages, and challenges of mEDC in data collection, project management, and telemonitoring in a randomized controlled trial (RCT). Methods: We developed an mEDC to support an RCT called ªTelmisartan and Hydrochlorothiazide Antihypertensive Treatment (THAT)º study, which was a multicenter, double-blinded, RCT, with the purpose of comparing the efficacy of telmisartan and hydrochlorothiazide (HCTZ) monotherapy in high-sodium-intake patients with mild to moderate hypertension during a 60 days follow-up. Semistructured interviews were conducted during and after the trial to evaluate the feasibility, advantage, and challenge of mEDC. Nvivo version 9.0 (QSR International) was used to analyze records of interviews, and a thematic framework method was used to obtain outcomes. -

Introduction 1
Notes Introduction 1. Amartya Sen’s Poverty and Famines remains the standard analy sis of the human and institutional causes of starvation. 2. For example, Arthur Waldron quotes Chen Duxiu, “Dao Junfa” (Down with war- lords), Meizhou pinglun, 28 December 1918 (“The Warlord,” 1080). 3. Sheridan, Chinese Warlord; Gillin, Warlord; A. Nathan, Peking Politics; Ch’i, War- lord Politics in China; McCormack, Chang Tso- lin; Wou, Militarism in Modern China. 4. Suleski, Civil Government in Warlord China, 190. This is not to say that under- standings of military figures in the republic have not evolved. After spending de cades as the “ running dogs of imperialism” in the Maoist period, more than a few warlords enjoyed something of a rehabilitation as local heroes in the 1980s, even as “something close to national heroes,” praised in the mainland Chinese press as “patriotic generals” for their roles in anti- Japanese re sis tance (Lary, foreword, vii– viii). 5. “Simply defined,” in the words of McCord, “warlords are military commanders who, as a result of their control of military force, exert a significant degree of au- tonomous po liti cal power within weak and fragmented po liti cal orders” (Military Force and Elite Power, 50). He explains elsewhere that as a system “warlordism did not originate simply in the rejection of legitimate po liti cal authority by military commanders, but rather in the difficulty of defining which authority was legiti- mate” (The Power of a Gun, 310). 6. Taking the form of both academic study and more popu lar history, this continuing trend is summarized by Zhang Qiang and Weatherley, “The Rise of ‘Republican Fever.’ ” 7.