Hedera Helix) on the Arthropod Community of Stanley Park
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biology and Ecology of Leptographium Species and Their Vectos As Components of Loblolly Pine Decline Lori G
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline Lori G. Eckhardt Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Plant Sciences Commons Recommended Citation Eckhardt, Lori G., "Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline" (2003). LSU Doctoral Dissertations. 2133. https://digitalcommons.lsu.edu/gradschool_dissertations/2133 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. BIOLOGY AND ECOLOGY OF LEPTOGRAPHIUM SPECIES AND THEIR VECTORS AS COMPONENTS OF LOBLOLLY PINE DECLINE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Plant Pathology & Crop Physiology by Lori G. Eckhardt B.S., University of Maryland, 1997 August 2003 © Copyright 2003 Lori G. Eckhardt All rights reserved ii ACKNOWLEDGMENTS I gratefully acknowledge the invaluable input provided by my dissertation advisor, Dr. John P. Jones. Among many other things, he has demonstrated his patients, enthusiasm and understanding as I struggled to pursue my graduate studies. I am indebted to Dr. Marc A. Cohn, for his guidance, encouragement, support and most of all, his friendship. -

Title Ecological Studies on Dispersal Flight and Host Selection of The
Ecological studies on dispersal flight and host selection of the Title ambrosia beetle Platypus quercivorus (Murayama)( Dissertation_全文 ) Author(s) Pham, Duy Long Citation 京都大学 Issue Date 2020-09-23 URL https://doi.org/10.14989/doctor.k22787 許諾条件により本文は2021-04-01に公開; Pham DL, Ito Y, Okada R, Ikeno H, Isagi Y, Yamasaki M (2017) Phototactic behavior of the ambrosia beetle Platypus quercivorus (Murayama) (Coleoptera: Platypodidae) before and after flight. Journal of Insect Behavior 30(3): 318-330. doi: Right 10.1007/s10905-017-9615-3. Pham DL, Ito Y, Okada R, Ikeno H, Isagi Y, Yamasaki M (2019) Effects of leaf conditions and flight activity on the behaviour of Platypus quercivorus (Murayama) (Coleoptera: Platypodidae). Journal of Applied Entomology 143(9): 1000-1010. doi: 10.1111/jen.12671 Type Thesis or Dissertation Textversion ETD Kyoto University Ecological studies on dispersal flight and host selection of the ambrosia beetle Platypus quercivorus (Murayama) Pham Duy Long 2020 Table of contents Chapter 1: General introduction: Dispersal flight and host selection of bark and ambrosia beetles ........................................................................................................................... 1 Chapter 2: Factors affecting flight distance of Platypus quercivorus ............................ 19 Chapter 3: Effects of flight on phototactic behavior of Platypus quercivorus .............. 35 Chapter 4: Effects of leaf conditions on the olfactory response of Platypus quercivorus to leaf volatiles ........................................................................................................... -

R. L. Meagher, Jr.R and J. C. Kgaspi 2 Texas A&M University Agricultural
S O U T IIW E S T E R N E N T O M O L O G IS T N IA R .2003 M ttIIN ‐F IE L D D IST R IB U T IO N O F T H R E E H O M O PT E R A N SP E C ttS IN T E X A S SU G A R C A N E R. L. Meagher,Jr.r andJ. C. kgaspi 2 TexasA&M University Agricultural Researchand ExtensionCenter 2415EastHighway 83 Weslaco,Texas 78596 ABSTRACT Sugarcanefields composed ofeither'CP 70-321'or'NCo 310'weresampled during 1993 and 1994 for three speciesofhomopteran insects. The West Indian canefly, Saccharosydne saccharivora(tlestwood), wasthe mostabundant speiies collectedwith densitiesreaching over 40 per shoot. Populationdensities varied sigrificantly throughoutthe seasonin 1993but were similar in 1994. No differencein densitieswere found between sugarcane cultivars. Both the sugarcanedelphacid, Perhinsiella saccharicidaKirkaldyand the leafhopperDraeculacephala portola Ball, were found in low numbers(< 1.0per shoot). P. saccharicidareached its highest levelslater in the season,and 'CP 70-321'shoots harbored more individuals than 'NCo 310' shoots.Although D. portola dertsitieswere low, differe,ncesamong fields andbetwecn cultivars were found. Aggregation,as measuredusing Taylor a and b coefficients, was shown to be greaterfor ,S.saccharivora than the other species. INTRODUCTION Sugarcane(interspecific hybrids of Saccharum)has been commercially grown in a tlree- county areaof southemTexas since 1972. Stemboringpyralids, Mexican rice borer, Eoreuma loftini (Dyar), andsugarcane borer, Diatraea saccharalis(F.), havebeen the most seriousinsect pests of the in{ustry (Meagher et al. 1994); however, other insects are active in the Texas sugarcaneagroecos)Nstem. -

Rove Beetles of Florida, Staphylinidae (Insecta: Coleoptera: Staphylinidae)1 J
EENY115 Rove Beetles of Florida, Staphylinidae (Insecta: Coleoptera: Staphylinidae)1 J. Howard Frank and Michael C. Thomas2 Introduction body form is much broader and the elytra almost cover (Scaphidiinae) or do cover (Scydmaenidae) the abdomen. Rove beetles are often abundant in habitats with large In most, the antennae are simple and typically have 11 numbers of fly larvae—especially decaying fruit, decaying antennomeres (“segments”), but in some (Pselaphinae) the seaweed, compost, carrion, and dung—where some are antennae are clubbed or (Micropeplinae) have a greatly important predators of maggots and others prey on mites or enlarged apical segment, or (some Aleocharinae) have 10 nematodes. Because they are abundant in decaying plants or (some Pselaphinae) even fewer antennomeres. Antennae and fruits, plant inspectors encounter them but often do are geniculate (“elbowed”) in a few members of Pselaphinae, not recognize them as beetles. This article is intended as Osoriinae, Oxytelinae, Paederinae, and Staphylininae. an introduction to the Florida representatives of this large, diverse, and important family of beetles. Characterization Adults range from less than 1 mm to 40 mm long (none here is to the level of subfamily (at least 18 subfamilies is more than about 20 mm in Florida), although almost occur in Florida) because characterization to the level all are less than about 7 mm long. Adults of some other of genus (or species) would be too complicated for a families also have short elytra, but in these (e.g., various publication of this kind. The best popular North American Histeridae; Limulodes and other Ptiliidae; Nicrophorus, identification guide to beetles (White 1983), likewise family Silphidae; Trypherus, family Cantharidae; Conotelus, characterizes Staphylinidae only to the level of subfamily family Nitidulidae; Rhipidius, family Rhipiphoridae; Meloe, (and its classification is outdated, and it does not provide family Meloidae; and Inopeplus, family Salpingidae) the references to the literature). -

Conifer Bole Utilization by Wood-Boring Beetles in Western Oregon Cfatc75
CfAtc75-;,) ?-437`,, ) Conifer bole utilization by wood-boring beetles in western Oregon /p i H. ZHONG AND T. D. SCHOWALTER1 Department of Entomology, Oregon State University, Corvallis, OR 97331, U.S.A. Received December 7, 1988 Accepted April 4, 1989 ZHONG, H., and SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19: 943-947. We studied wood excavation by scolytid and cerambycid beetles in decomposing boles of four conifer species during the first two years on the ground in western Oregon. Colonization density and gallery volumes were measured in experi- mental boles (0.5 m diameter x 5 m length) of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), western hemlock (Tsuga heterophylla (Raf.) Sarg.), Pacific silver fir (Abies amabilis (Dougl.) Forbes), and western red cedar ( Thuja plicata Donn). Ambrosia beetles (Coleoptera: Scolytidae) colonized boles only during the 1st year and were essentially restricted to Douglas-fir and western hemlock (removing 0.2% of the sapwood volume). Bark beetles (Coleoptera: Scolytidae) colonized boles only in the 1st year, primarily in Douglas-fir and Pacific silver fir (removing 7-8% of the phloem surface area). Wood borers (Coleoptera: Cerambycidae) excavated an additional 2.3% of the phloem surface area of Pacific silver fir in the 1st year and continued to excavate all species except Douglas-fir during the 2nd year. Consequences for the decomposition process are discussed. ZHONG, H., et SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19 : 943-947. Lexcavation du bois par les coleopteres dans les troncs en decomposition de quatre especes de coniferes a ete etudiee durant les 2 premieres annees de leur position au sol dans louest de ]Oregon. -
Draft Clearwater Assessment: 5. Vegetative Resources
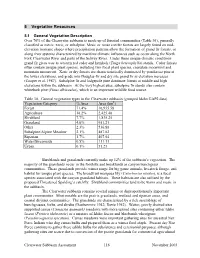
5 Vegetative Resources 5.1 General Vegetation Description Over 70% of the Clearwater subbasin is made up of forested communities (Table 30), generally classified as mesic, xeric, or subalpine. Mesic or moist conifer forests are largely found on mid- elevation montane slopes where precipitation patterns allow the formation of grand fir forests, or along river systems characterized by maritime climatic influences such as occur along the North Fork Clearwater River and parts of the Selway River. Under these unique climatic conditions grand fir gives way to western red cedar and hemlock (Tsuga heterophylla) stands. Cedar forests often contain unique plant species, including two focal plant species, crenulate moonwort and mountain moonwort. Xeric or dry forests are characteristically dominated by ponderosa pine at the lower elevations, and grade into Douglas-fir and dry site grand fir as elevation increases (Cooper et al. 1987). Subalpine fir and lodgepole pine dominate forests at middle and high elevations within the subbasin. At the very highest sites, subalpine fir stands also contain whitebark pine (Pinus albicaulus), which is an important wildlife food source. Table 30. General vegetation types in the Clearwater subbasin (grouped Idaho GAP2 data) Vegetation Category % Area Area (km2) Forest 71.4% 16,955.58 Agriculture 10.2% 2,425.48 Shrubland 7.7% 1,835.25 Grassland 4.0% 951.21 Other 2.3% 536.88 Subalpine/Alpine Meadow 2.1% 487.02 Riparian 1.7% 407.04 Water/Streamside 0.5% 111.11 Urban 0.1% 31.23 Shrublands and grasslands currently make up 12% of the subbasin’s vegetation. The majority of the grasslands occur in the foothills and breaklands as canyon bunchgrass communities. -

Effects of Pathogens and Bark Beetles on Forests
Utah State University DigitalCommons@USU Quinney Natural Resources Research Library, The Bark Beetles, Fuels, and Fire Bibliography S.J. and Jessie E. 1993 Effects of Pathogens and Bark Beetles on Forests D J. Goheen E M. Hansen Follow this and additional works at: https://digitalcommons.usu.edu/barkbeetles Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Forest Biology Commons, Forest Management Commons, and the Wood Science and Pulp, Paper Technology Commons Recommended Citation Goheen, D. and Hansen, E. Effects of pathogens and bark beetles on forests, pp. 175-196 in: TD Schowalter & GM Filip (eds) Beetle-Pathogen Interactions in Conifer Forests, Academic Press, London. This Contribution to Book is brought to you for free and open access by the Quinney Natural Resources Research Library, S.J. and Jessie E. at DigitalCommons@USU. It has been accepted for inclusion in The Bark Beetles, Fuels, and Fire Bibliography by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. .... 9.... Effects of Pathogens and Bark Beetles on Forests D. J. GOHEEN1 and E. M. HANSEN2 1USDA Forest Service, Pacific Northwest Region, Portland, OR, USA 2Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, USA 9.1 Introduction 175 9.2 Effects of Bark Beetle-Pathogen Interactions 176 9.2.1 Interactions in Pseudotsuga Forests: Phellinus and Deruiroctonus 176 9.2.2 Interactions in Pseudotsuga Forests: Leptographium and Bark Beetles 180 9.2.3 Interactions in Pinus ponderosa Forests 183 9.2.4 Interactions in Pinus contorta Forests 186 9.2.5 Interactions in Abies Forests 187 9.2.6 Interactions in Eastern Forests 190 9.3 Conclusions 191 References 191 9.1 INTRODUCTION Pathogenic fungi and bark beetles are important components of most coniferous forest ecosystems. -

A) Coleoptera: Curculionidae (Weevils) Family Characteristics: One of the Largest Animal Families with ~90,000 Described Species
A) Coleoptera: Curculionidae (weevils) Family characteristics: One of the largest animal families with ~90,000 described species. While there is considerable diversity in form and size, weevils have distinctive long snouts with chewing mouthparts at the end B and clubbed antennae. Steremnius carinatus A Conifer seedling weevil Economic importance: Adults feed at the base of vegetation (E). Clear cuts and site preparation encourage the weevil to feed on conifer seedlings. Economic injury has been worst on Vancouver Island and Haida Gwaii. Principal hosts: Seedlings of Douglas-fir, Sitka spruce C Hemlock and true firs are less preferred but sometimes attacked. 1mm Signs in the field: 1. Pupal cells at the base of seedlings (B, D) Notes : D E Adult Body Antennae Special Feature Injury Long and slender snout (C) Club shaped Flightless, adults Adult is injurious stage and obvious walk from host to Red-grey patterns on elytra Emerge from stumps and girdle host 7 – 10mm long seedlings (E) Larvae Body Injury Legless, curved (A) Larvae live in bark of stumps and slash from recent felling, where they feed on phloem White to pink, with brown head capsule Strong mandibles Images by: A), B) Terry Price, Georgia Forestry Commission, Bugwood.org C) USDA Forest Service - Northeastern Area Archive, USDA Forest Service, Bugwood.org D), E) http://www.forestry.ubc.ca/fetch21/FRST308/lab3/steremnius_carinatus/collar.html 1 Hylobius warreni A B Warren root collar weevil Economic importance: Feeding by larvae may cause girdling and subsequent tree mortality, as well as serious reductions in growth. May seriously hamper the establishment of pine D plantations. -

Standardised Arthropod (Arthropoda) Inventory Across Natural and Anthropogenic Impacted Habitats in the Azores Archipelago
Biodiversity Data Journal 9: e62157 doi: 10.3897/BDJ.9.e62157 Data Paper Standardised arthropod (Arthropoda) inventory across natural and anthropogenic impacted habitats in the Azores archipelago José Marcelino‡, Paulo A. V. Borges§,|, Isabel Borges ‡, Enésima Pereira§‡, Vasco Santos , António Onofre Soares‡ ‡ cE3c – Centre for Ecology, Evolution and Environmental Changes / Azorean Biodiversity Group and Universidade dos Açores, Rua Madre de Deus, 9500, Ponta Delgada, Portugal § cE3c – Centre for Ecology, Evolution and Environmental Changes / Azorean Biodiversity Group and Universidade dos Açores, Rua Capitão João d’Ávila, São Pedro, 9700-042, Angra do Heroismo, Portugal | IUCN SSC Mid-Atlantic Islands Specialist Group, Angra do Heroísmo, Portugal Corresponding author: Paulo A. V. Borges ([email protected]) Academic editor: Pedro Cardoso Received: 17 Dec 2020 | Accepted: 15 Feb 2021 | Published: 10 Mar 2021 Citation: Marcelino J, Borges PAV, Borges I, Pereira E, Santos V, Soares AO (2021) Standardised arthropod (Arthropoda) inventory across natural and anthropogenic impacted habitats in the Azores archipelago. Biodiversity Data Journal 9: e62157. https://doi.org/10.3897/BDJ.9.e62157 Abstract Background In this paper, we present an extensive checklist of selected arthropods and their distribution in five Islands of the Azores (Santa Maria. São Miguel, Terceira, Flores and Pico). Habitat surveys included five herbaceous and four arboreal habitat types, scaling up from native to anthropogenic managed habitats. We aimed to contribute -

FIELD GUIDE to FOREST DAMAGE in British Columbia
FIELD GUIDE TO FOREST DAMAGE in British Columbia 3RD REVISED EDITION Field Guide to the Pests of Managed Forests in British Columbia (1983) Canadian Cataloguing in Publication Data The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the Government of British Columbia of any product or service to the exclusion of any others that may also be suitable. Contents of this report are presented for discussion purposes only. Funding assistance does not imply endorsement of any statements or information contained herein by the Government of British Columbia. Uniform Resource Locators (urls), addresses, and contact information contained in this document are current at the time of printing unless otherwise noted. Print edition: ISBN 978-0-7726-6819-6 Electronic/PDF edition: ISBN 978-0-7726-6819-6 Citation Burleigh, J., T. Ebata, K.J. White, D. Rusch and H. Kope. (Eds.) 2014. Field Guide to Forest Damage in British Columbia (Joint publication, ISSN 0843-4719 ; no. 17) Authors’ affiliation Jennifer Burleigh, Tim Ebata and Harry Kope B.C. Ministry of Forests, Lands and Natural Resource Operations Resource Practices Branch, Victoria, B.C. Ken White B.C. Ministry of Forests, Lands and Natural Resource Operations Skeena Region, Smithers, B.C. David Rusch B.C. Ministry of Forests, Lands and Natural Resource Operations Cariboo Region, Williams Lake, B.C. Copies of this report may be obtained from: Crown Publications, Queen’s Printer PO Box 9452 Stn Prov Govt Victoria, BC v8w 9v7 1-800-663-6105 | www.crownpub.bc.ca For information on other publications in this series, visit www.for.gov.bc.ca/scripts/hfd/pubs/hfdcatalog/index.asp © 2014 Province of British Columbia When using information from this report, please cite fully and correctly. -

Present Taxonomic Work on Staphylinidae (Coleoptera) in Canada: Progress Against All Odds
A peer-reviewed open-access journal ZooKeys 186: 1–5 (2012) Present taxonomic work on Staphylinidae in Canada 1 doi: 10.3897/zookeys.186.3252 EDITORIAL www.zookeys.org Launched to accelerate biodiversity research Present taxonomic work on Staphylinidae (Coleoptera) in Canada: progress against all odds Adam Brunke1, Jan Klimaszewski2, Robert S. Anderson3 1 Zoological Museum (Natural History Museum of Denmark), University of Copenhagen, 15 Universitetspar- ken, Copenhagen, Denmark, 2100 DK 2 Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre, 1055 du P.E.P.S., P.O. Box 10380, Stn. Sainte-Foy, Québec, Quebec, Canada G1V 4C7 3 Research Division, Canadian Museum of Nature, P.O. Box 3443, Ottawa, Ontario, Canada K1P 6P4 Corresponding author: Adam J. Brunke ([email protected]) Academic editor: L. Penev | Received 26 March 2012 | Accepted 23 April 2012 | Published 26 April 2012 Citation: Brunke A, Klimaszewski J, Anderson RS (2012) Present taxonomic work on Staphylinidae (Coleoptera) in Canada: progress against all odds. In: Klimaszewski J, Anderson R (Eds) Biosystematics and Ecology of Canadian Staphylinidae (Coleoptera) II. ZooKeys 186: 1–5. doi: 10.3897/zookeys.186.3252 The Staphylinidae or rove beetles represent one of the largest evolutionary radiations on earth with more than 56,000 species (Grebennikov and Newton 2009, Ślipiński et al. 2011) and are a dominant element of the insect fauna in many terrestrial ecosystems (examples discussed in Thayer 2005). Even though such an enormous diversity may seem unmanageable, we are living in an exciting time for taxonomic research on rove beetles. There has been a relatively sudden expansion in the field of bioinformatics, in- cluding e-taxonomy (see ZooKeys special issue 150 for further information). -

Western Redcedar
Thujaplicafa Donn ex D. Don Western Redcedar Cupressaceae Cypress family Don Minore Western redcedar (Thuja plicata), also called Pacific redcedar, giant-cedar, arborvitae, canoe-cedar, and shinglewood, is the only Thuja species native to western North America. Extant redcedar volumes are estimated to be 824 million m3 (29 billion R3) in British Columbia (43) and 228 million m3 (8 billion ft3) in the United States (16). Most of this volume is in mature trees, which have tapered, often-fluted bases, drooping branches, thin fibrous bark, and small scalelike leaves arrayed in flat sprays. Many have forked tops (fig. 1). They often reach ages of 800 to 1,000 years. One particularly large specimen in Washington has a d.b.h. of 592 cm (233 in), a height of 54.3 m (178 ft), and a crown spread of 16.5 m (54 ft). The wood is valuable and extensively used in a wide variety of products. Habitat Native Range Western redcedar grows along the Pacific coast from Humboldt County, CA (lat. 40” 10’ NJ, to the northern and western shores of Sumner Strait in southeastern Alaska (lat. 56” 30’ N.) (fig. 2). In California, it is common only in the lower Mad River drainage and the wet region south of Ferndale in Humboldt County; it is found elsewhere only in iso- lated stands in boggy habitats (19). North of the California-Oregon border, the coastal range broadens to include the western slopes of the Cas- cade Range north of Crater Lake and the eastern slopes north of about latitude 44” 30’ N.