Forest Insect & Disease Management Guide for the Northern and Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biology and Ecology of Leptographium Species and Their Vectos As Components of Loblolly Pine Decline Lori G
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline Lori G. Eckhardt Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Plant Sciences Commons Recommended Citation Eckhardt, Lori G., "Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline" (2003). LSU Doctoral Dissertations. 2133. https://digitalcommons.lsu.edu/gradschool_dissertations/2133 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. BIOLOGY AND ECOLOGY OF LEPTOGRAPHIUM SPECIES AND THEIR VECTORS AS COMPONENTS OF LOBLOLLY PINE DECLINE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Plant Pathology & Crop Physiology by Lori G. Eckhardt B.S., University of Maryland, 1997 August 2003 © Copyright 2003 Lori G. Eckhardt All rights reserved ii ACKNOWLEDGMENTS I gratefully acknowledge the invaluable input provided by my dissertation advisor, Dr. John P. Jones. Among many other things, he has demonstrated his patients, enthusiasm and understanding as I struggled to pursue my graduate studies. I am indebted to Dr. Marc A. Cohn, for his guidance, encouragement, support and most of all, his friendship. -

Heterobasidion Annosum (Hunt and Cobb 1982)
Interactions of Root Disease and Bark Beetles1 George T. Ferrell J. Richard Parmeter, Jr.2 Abstract: Associations between root diseases (Castello and others 1976; Harrington 1980), and bark beetles (Scolytidae) constitute some which may explain the rapid decay of sapwood of the most serious pest complexes affecting following tree-killing by bark beetles. forests in North America and elsewhere. The Despite much research, vectoring of root interactive functioning of these pests disease fungi by stem-infesting bark beetles derives from the following relationships: 1) has not yet been firmly established, but they root diseases predispose trees to bark beetle have been implicated as vectors of Peniophora infestation by lowering resistance, and gigantea (Fr.) Jul., a fungal antagonist of perhaps increasing attractiveness, of trees Heterobasidion annosum (Hunt and Cobb 1982). to the attacking beetles; 2) bark beetles may Recently, however, a species of be vectors of root disease fungi or may root-colonizing bark beetle in the genus create infection courts for them. Hylastes and root-colonizing species of the weevil genera Steremnius and Pissodes have been implicated in vectoring Leptographium (Verticicladiella) wageneri (Kendr.) Wingf., the fungus causing black-stain root disease Symbioses between bark beetles and fungi in Douglas-fir (Harrington and others 1985, are common in nature, and previous reviewers Witcosky and others 1986a). As these vectors (e.g., Graham 1967) have identified several readily breed in roots of recently cut types of these symbiotic relationships, some stumps, their populations evidently increase of which appear to be beneficial to both in stands after thinning (Harrington and (mutualism), and others which apparently others 1985; Witcosky and others 1986b). -

Title Ecological Studies on Dispersal Flight and Host Selection of The
Ecological studies on dispersal flight and host selection of the Title ambrosia beetle Platypus quercivorus (Murayama)( Dissertation_全文 ) Author(s) Pham, Duy Long Citation 京都大学 Issue Date 2020-09-23 URL https://doi.org/10.14989/doctor.k22787 許諾条件により本文は2021-04-01に公開; Pham DL, Ito Y, Okada R, Ikeno H, Isagi Y, Yamasaki M (2017) Phototactic behavior of the ambrosia beetle Platypus quercivorus (Murayama) (Coleoptera: Platypodidae) before and after flight. Journal of Insect Behavior 30(3): 318-330. doi: Right 10.1007/s10905-017-9615-3. Pham DL, Ito Y, Okada R, Ikeno H, Isagi Y, Yamasaki M (2019) Effects of leaf conditions and flight activity on the behaviour of Platypus quercivorus (Murayama) (Coleoptera: Platypodidae). Journal of Applied Entomology 143(9): 1000-1010. doi: 10.1111/jen.12671 Type Thesis or Dissertation Textversion ETD Kyoto University Ecological studies on dispersal flight and host selection of the ambrosia beetle Platypus quercivorus (Murayama) Pham Duy Long 2020 Table of contents Chapter 1: General introduction: Dispersal flight and host selection of bark and ambrosia beetles ........................................................................................................................... 1 Chapter 2: Factors affecting flight distance of Platypus quercivorus ............................ 19 Chapter 3: Effects of flight on phototactic behavior of Platypus quercivorus .............. 35 Chapter 4: Effects of leaf conditions on the olfactory response of Platypus quercivorus to leaf volatiles ........................................................................................................... -

R. L. Meagher, Jr.R and J. C. Kgaspi 2 Texas A&M University Agricultural
S O U T IIW E S T E R N E N T O M O L O G IS T N IA R .2003 M ttIIN ‐F IE L D D IST R IB U T IO N O F T H R E E H O M O PT E R A N SP E C ttS IN T E X A S SU G A R C A N E R. L. Meagher,Jr.r andJ. C. kgaspi 2 TexasA&M University Agricultural Researchand ExtensionCenter 2415EastHighway 83 Weslaco,Texas 78596 ABSTRACT Sugarcanefields composed ofeither'CP 70-321'or'NCo 310'weresampled during 1993 and 1994 for three speciesofhomopteran insects. The West Indian canefly, Saccharosydne saccharivora(tlestwood), wasthe mostabundant speiies collectedwith densitiesreaching over 40 per shoot. Populationdensities varied sigrificantly throughoutthe seasonin 1993but were similar in 1994. No differencein densitieswere found between sugarcane cultivars. Both the sugarcanedelphacid, Perhinsiella saccharicidaKirkaldyand the leafhopperDraeculacephala portola Ball, were found in low numbers(< 1.0per shoot). P. saccharicidareached its highest levelslater in the season,and 'CP 70-321'shoots harbored more individuals than 'NCo 310' shoots.Although D. portola dertsitieswere low, differe,ncesamong fields andbetwecn cultivars were found. Aggregation,as measuredusing Taylor a and b coefficients, was shown to be greaterfor ,S.saccharivora than the other species. INTRODUCTION Sugarcane(interspecific hybrids of Saccharum)has been commercially grown in a tlree- county areaof southemTexas since 1972. Stemboringpyralids, Mexican rice borer, Eoreuma loftini (Dyar), andsugarcane borer, Diatraea saccharalis(F.), havebeen the most seriousinsect pests of the in{ustry (Meagher et al. 1994); however, other insects are active in the Texas sugarcaneagroecos)Nstem. -

Biodiversity and Coarse Woody Debris in Southern Forests Proceedings of the Workshop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity
Biodiversity and Coarse woody Debris in Southern Forests Proceedings of the Workshop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity Athens, GA - October 18-20,1993 Biodiversity and Coarse Woody Debris in Southern Forests Proceedings of the Workhop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity Athens, GA October 18-20,1993 Editors: James W. McMinn, USDA Forest Service, Southern Research Station, Forestry Sciences Laboratory, Athens, GA, and D.A. Crossley, Jr., University of Georgia, Athens, GA Sponsored by: U.S. Department of Energy, Savannah River Site, and the USDA Forest Service, Savannah River Forest Station, Biodiversity Program, Aiken, SC Conducted by: USDA Forest Service, Southem Research Station, Asheville, NC, and University of Georgia, Institute of Ecology, Athens, GA Preface James W. McMinn and D. A. Crossley, Jr. Conservation of biodiversity is emerging as a major goal in The effects of CWD on biodiversity depend upon the management of forest ecosystems. The implied harvesting variables, distribution, and dynamics. This objective is the conservation of a full complement of native proceedings addresses the current state of knowledge about species and communities within the forest ecosystem. the influences of CWD on the biodiversity of various Effective implementation of conservation measures will groups of biota. Research priorities are identified for future require a broader knowledge of the dimensions of studies that should provide a basis for the conservation of biodiversity, the contributions of various ecosystem biodiversity when interacting with appropriate management components to those dimensions, and the impact of techniques. management practices. We thank John Blake, USDA Forest Service, Savannah In a workshop held in Athens, GA, October 18-20, 1993, River Forest Station, for encouragement and support we focused on an ecosystem component, coarse woody throughout the workshop process. -

Conifer Bole Utilization by Wood-Boring Beetles in Western Oregon Cfatc75
CfAtc75-;,) ?-437`,, ) Conifer bole utilization by wood-boring beetles in western Oregon /p i H. ZHONG AND T. D. SCHOWALTER1 Department of Entomology, Oregon State University, Corvallis, OR 97331, U.S.A. Received December 7, 1988 Accepted April 4, 1989 ZHONG, H., and SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19: 943-947. We studied wood excavation by scolytid and cerambycid beetles in decomposing boles of four conifer species during the first two years on the ground in western Oregon. Colonization density and gallery volumes were measured in experi- mental boles (0.5 m diameter x 5 m length) of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), western hemlock (Tsuga heterophylla (Raf.) Sarg.), Pacific silver fir (Abies amabilis (Dougl.) Forbes), and western red cedar ( Thuja plicata Donn). Ambrosia beetles (Coleoptera: Scolytidae) colonized boles only during the 1st year and were essentially restricted to Douglas-fir and western hemlock (removing 0.2% of the sapwood volume). Bark beetles (Coleoptera: Scolytidae) colonized boles only in the 1st year, primarily in Douglas-fir and Pacific silver fir (removing 7-8% of the phloem surface area). Wood borers (Coleoptera: Cerambycidae) excavated an additional 2.3% of the phloem surface area of Pacific silver fir in the 1st year and continued to excavate all species except Douglas-fir during the 2nd year. Consequences for the decomposition process are discussed. ZHONG, H., et SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19 : 943-947. Lexcavation du bois par les coleopteres dans les troncs en decomposition de quatre especes de coniferes a ete etudiee durant les 2 premieres annees de leur position au sol dans louest de ]Oregon. -
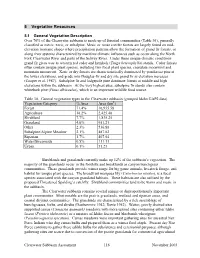
Draft Clearwater Assessment: 5. Vegetative Resources
5 Vegetative Resources 5.1 General Vegetation Description Over 70% of the Clearwater subbasin is made up of forested communities (Table 30), generally classified as mesic, xeric, or subalpine. Mesic or moist conifer forests are largely found on mid- elevation montane slopes where precipitation patterns allow the formation of grand fir forests, or along river systems characterized by maritime climatic influences such as occur along the North Fork Clearwater River and parts of the Selway River. Under these unique climatic conditions grand fir gives way to western red cedar and hemlock (Tsuga heterophylla) stands. Cedar forests often contain unique plant species, including two focal plant species, crenulate moonwort and mountain moonwort. Xeric or dry forests are characteristically dominated by ponderosa pine at the lower elevations, and grade into Douglas-fir and dry site grand fir as elevation increases (Cooper et al. 1987). Subalpine fir and lodgepole pine dominate forests at middle and high elevations within the subbasin. At the very highest sites, subalpine fir stands also contain whitebark pine (Pinus albicaulus), which is an important wildlife food source. Table 30. General vegetation types in the Clearwater subbasin (grouped Idaho GAP2 data) Vegetation Category % Area Area (km2) Forest 71.4% 16,955.58 Agriculture 10.2% 2,425.48 Shrubland 7.7% 1,835.25 Grassland 4.0% 951.21 Other 2.3% 536.88 Subalpine/Alpine Meadow 2.1% 487.02 Riparian 1.7% 407.04 Water/Streamside 0.5% 111.11 Urban 0.1% 31.23 Shrublands and grasslands currently make up 12% of the subbasin’s vegetation. The majority of the grasslands occur in the foothills and breaklands as canyon bunchgrass communities. -

Effects of Pathogens and Bark Beetles on Forests
Utah State University DigitalCommons@USU Quinney Natural Resources Research Library, The Bark Beetles, Fuels, and Fire Bibliography S.J. and Jessie E. 1993 Effects of Pathogens and Bark Beetles on Forests D J. Goheen E M. Hansen Follow this and additional works at: https://digitalcommons.usu.edu/barkbeetles Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Forest Biology Commons, Forest Management Commons, and the Wood Science and Pulp, Paper Technology Commons Recommended Citation Goheen, D. and Hansen, E. Effects of pathogens and bark beetles on forests, pp. 175-196 in: TD Schowalter & GM Filip (eds) Beetle-Pathogen Interactions in Conifer Forests, Academic Press, London. This Contribution to Book is brought to you for free and open access by the Quinney Natural Resources Research Library, S.J. and Jessie E. at DigitalCommons@USU. It has been accepted for inclusion in The Bark Beetles, Fuels, and Fire Bibliography by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. .... 9.... Effects of Pathogens and Bark Beetles on Forests D. J. GOHEEN1 and E. M. HANSEN2 1USDA Forest Service, Pacific Northwest Region, Portland, OR, USA 2Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, USA 9.1 Introduction 175 9.2 Effects of Bark Beetle-Pathogen Interactions 176 9.2.1 Interactions in Pseudotsuga Forests: Phellinus and Deruiroctonus 176 9.2.2 Interactions in Pseudotsuga Forests: Leptographium and Bark Beetles 180 9.2.3 Interactions in Pinus ponderosa Forests 183 9.2.4 Interactions in Pinus contorta Forests 186 9.2.5 Interactions in Abies Forests 187 9.2.6 Interactions in Eastern Forests 190 9.3 Conclusions 191 References 191 9.1 INTRODUCTION Pathogenic fungi and bark beetles are important components of most coniferous forest ecosystems. -

A) Coleoptera: Curculionidae (Weevils) Family Characteristics: One of the Largest Animal Families with ~90,000 Described Species
A) Coleoptera: Curculionidae (weevils) Family characteristics: One of the largest animal families with ~90,000 described species. While there is considerable diversity in form and size, weevils have distinctive long snouts with chewing mouthparts at the end B and clubbed antennae. Steremnius carinatus A Conifer seedling weevil Economic importance: Adults feed at the base of vegetation (E). Clear cuts and site preparation encourage the weevil to feed on conifer seedlings. Economic injury has been worst on Vancouver Island and Haida Gwaii. Principal hosts: Seedlings of Douglas-fir, Sitka spruce C Hemlock and true firs are less preferred but sometimes attacked. 1mm Signs in the field: 1. Pupal cells at the base of seedlings (B, D) Notes : D E Adult Body Antennae Special Feature Injury Long and slender snout (C) Club shaped Flightless, adults Adult is injurious stage and obvious walk from host to Red-grey patterns on elytra Emerge from stumps and girdle host 7 – 10mm long seedlings (E) Larvae Body Injury Legless, curved (A) Larvae live in bark of stumps and slash from recent felling, where they feed on phloem White to pink, with brown head capsule Strong mandibles Images by: A), B) Terry Price, Georgia Forestry Commission, Bugwood.org C) USDA Forest Service - Northeastern Area Archive, USDA Forest Service, Bugwood.org D), E) http://www.forestry.ubc.ca/fetch21/FRST308/lab3/steremnius_carinatus/collar.html 1 Hylobius warreni A B Warren root collar weevil Economic importance: Feeding by larvae may cause girdling and subsequent tree mortality, as well as serious reductions in growth. May seriously hamper the establishment of pine D plantations. -

FIELD GUIDE to FOREST DAMAGE in British Columbia
FIELD GUIDE TO FOREST DAMAGE in British Columbia 3RD REVISED EDITION Field Guide to the Pests of Managed Forests in British Columbia (1983) Canadian Cataloguing in Publication Data The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the Government of British Columbia of any product or service to the exclusion of any others that may also be suitable. Contents of this report are presented for discussion purposes only. Funding assistance does not imply endorsement of any statements or information contained herein by the Government of British Columbia. Uniform Resource Locators (urls), addresses, and contact information contained in this document are current at the time of printing unless otherwise noted. Print edition: ISBN 978-0-7726-6819-6 Electronic/PDF edition: ISBN 978-0-7726-6819-6 Citation Burleigh, J., T. Ebata, K.J. White, D. Rusch and H. Kope. (Eds.) 2014. Field Guide to Forest Damage in British Columbia (Joint publication, ISSN 0843-4719 ; no. 17) Authors’ affiliation Jennifer Burleigh, Tim Ebata and Harry Kope B.C. Ministry of Forests, Lands and Natural Resource Operations Resource Practices Branch, Victoria, B.C. Ken White B.C. Ministry of Forests, Lands and Natural Resource Operations Skeena Region, Smithers, B.C. David Rusch B.C. Ministry of Forests, Lands and Natural Resource Operations Cariboo Region, Williams Lake, B.C. Copies of this report may be obtained from: Crown Publications, Queen’s Printer PO Box 9452 Stn Prov Govt Victoria, BC v8w 9v7 1-800-663-6105 | www.crownpub.bc.ca For information on other publications in this series, visit www.for.gov.bc.ca/scripts/hfd/pubs/hfdcatalog/index.asp © 2014 Province of British Columbia When using information from this report, please cite fully and correctly. -

Western Redcedar
Thujaplicafa Donn ex D. Don Western Redcedar Cupressaceae Cypress family Don Minore Western redcedar (Thuja plicata), also called Pacific redcedar, giant-cedar, arborvitae, canoe-cedar, and shinglewood, is the only Thuja species native to western North America. Extant redcedar volumes are estimated to be 824 million m3 (29 billion R3) in British Columbia (43) and 228 million m3 (8 billion ft3) in the United States (16). Most of this volume is in mature trees, which have tapered, often-fluted bases, drooping branches, thin fibrous bark, and small scalelike leaves arrayed in flat sprays. Many have forked tops (fig. 1). They often reach ages of 800 to 1,000 years. One particularly large specimen in Washington has a d.b.h. of 592 cm (233 in), a height of 54.3 m (178 ft), and a crown spread of 16.5 m (54 ft). The wood is valuable and extensively used in a wide variety of products. Habitat Native Range Western redcedar grows along the Pacific coast from Humboldt County, CA (lat. 40” 10’ NJ, to the northern and western shores of Sumner Strait in southeastern Alaska (lat. 56” 30’ N.) (fig. 2). In California, it is common only in the lower Mad River drainage and the wet region south of Ferndale in Humboldt County; it is found elsewhere only in iso- lated stands in boggy habitats (19). North of the California-Oregon border, the coastal range broadens to include the western slopes of the Cas- cade Range north of Crater Lake and the eastern slopes north of about latitude 44” 30’ N. -

Soil Arthropods in the Central Cascades: Slash Burning Effects and Biology of Some Species
AN ABSTRACT OF THE THESIS OF Edith G. Estrada-Venegas for the degree of Master in Science in Entomology presented on May 1, 1995. Title: Soil Arthropods in the Central Cascades: Slash Burning Effects and Biology of Some Species. Redacted for Privacy Abstract approved: Andrew R. Moldenke arid Gerald W. Krantz Despite the recognized role of soil arthropod fauna on nutrient cycling and decomposition processes, many aspects of the effects of sylvicultural methods in forest ecosystems upon their biology remain poorly understood. The long term effects of prescribed fires on soil arthropods in forest ecosystems in the Pacific Northwest have never been studied. Soil samples were taken from three sites located in the Willamette National Forest in 1992: paired sites that were either clear-cut without burning and clear-cut with burning 40 years ago. One hundred and eight samples were processed; the arthropods were separated, identified and counted. To study the biology and behavior of some arthropods, eight species of oribatid mites were reared in laboratory conditions. Their life cycle, feeding behavior and reproduction were studied. Results indicated that there were no statistical significant treatment differences either in terms of total numbers of organisms or biomass. However, the majority of the commonest taxa did show offsetting treatment responses. A total of 204 taxa were found in the three sites. The most important groups included Collembola, mites, and insects. Other groups also represented, but in smaller numbers, were spiders, symphylans, pseudoscorpions, and centipedes. Of all these groups, oribatid mites was the best represented and appears to be a useful indicator of disturbances.