Draft Clearwater Assessment: 5. Vegetative Resources
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thuja Plicata Has Many Traditional Uses, from the Manufacture of Rope to Waterproof Hats, Nappies and Other Kinds of Clothing
photograph © Daniel Mosquin Culturally modified tree. The bark of Thuja plicata has many traditional uses, from the manufacture of rope to waterproof hats, nappies and other kinds of clothing. Careful, modest, bark stripping has little effect on the health or longevity of trees. (see pages 24 to 35) photograph © Douglas Justice 24 Tree of the Year : Thuja plicata Donn ex D. Don In this year’s Tree of the Year article DOUGLAS JUSTICE writes an account of the western red-cedar or giant arborvitae (tree of life), a species of conifers that, for centuries has been central to the lives of people of the Northwest Coast of America. “In a small clearing in the forest, a young woman is in labour. Two women companions urge her to pull hard on the cedar bark rope tied to a nearby tree. The baby, born onto a newly made cedar bark mat, cries its arrival into the Northwest Coast world. Its cradle of firmly woven cedar root, with a mattress and covering of soft-shredded cedar bark, is ready. The young woman’s husband and his uncle are on the sea in a canoe carved from a single red-cedar log and are using paddles made from knot-free yellow cedar. When they reach the fishing ground that belongs to their family, the men set out a net of cedar bark twine weighted along one edge by stones lashed to it with strong, flexible cedar withes. Cedar wood floats support the net’s upper edge. Wearing a cedar bark hat, cape and skirt to protect her from the rain and INTERNATIONAL DENDROLOGY SOCIETY TREES Opposite, A grove of 80- to 100-year-old Thuja plicata in Queen Elizabeth Park, Vancouver. -

Dying Cedar Hedges —What Is the Cause?
Points covered in this factsheet Symptoms Planting problems Physiological effects Environmental, Soil and Climate factors Insect, Disease and Vertebrate agents Dying Cedar Hedges —What Is The Cause? Attractive and normally trouble free, cedar trees can be great additions to the landscape. Dieback of cedar hedging in the landscape is a common prob- lem. In most cases, it is not possible to pinpoint one single cause. Death is usually the result of a combination of envi- ronmental stresses, soil factors and problems originating at planting. Disease, insect or animal injury is a less frequent cause. Identifying The Host Certain species of cedar are susceptible to certain problems, so identifying the host plant can help to identify the cause and whether a symptom is an issue of concern or is normal for that plant. The most common columnar hedging cedars are Thuja plicata (Western Red Cedar - native to the West Coast) and Thuja occidentalis (American Arborvitae or Eastern White ICULTURE, PLANT HEALTH UNIT Cedar). Both species are often called arborvitae. Common varieties of Western Red ce- dar are ‘Emerald Giant’, ‘Excelsa’ and Atrovirens’. ‘Smaragd’ and ‘Pyramidalis’ are com- mon varieties of Eastern White cedar hedging. Species of Cupressus (Cypress), Chamaecyparis nootkatensis (Yellow Cedar or False Cypress) and Chamaecyparis law- soniana (Port Orford Cedar or lawsom Cypress) are also used in hedging. Symptoms The pattern of symptom development/distribution can provide a clue to whether the prob- lem is biotic (infectious) or abiotic (non-infectious). Trees often die out in a group, in one section of the hedge, or at random throughout the hedge. -

Final Environmental Impact Statement Nez Perce Tribal Hatchery Program
Final Environmental Impact Statement Nez Perce Tribal Hatchery Program Bonneville Power Administration U.S. Department of Energy Bureau of Indian Affairs U.S. Department of the Interior Nez Perce Tribe July 1997 Final Environmental Impact Statement Responsible Agencies: U.S. Department of Energy, Bonneville Power Administration (BPA); U.S. Department of the Interior, Bureau of Indian Affairs (BIA); Nez Perce Tribe (NPT). Title of Proposed Action: Nez Perce Tribal Hatchery Program. States Involved: Idaho. Abstract: Bonneville Power Administration, the Bureau of Indian Affairs, and the Nez Perce Tribe propose a supplementation program to restore chinook salmon to the Clearwater River Subbasin in Idaho. The Clearwater River is a tributary to the Snake River, which empties into the Columbia River. The Final EIS includes a new alternative suggested by commentors to the Draft EIS. In the Proposed Action, the Nez Perce Tribe would build and operate two central incubation and rearing hatcheries and six satellite facilities. Spring and fall chinook salmon would be reared and acclimated to different areas in the Subbasin and released at the hatchery and satellite sites or in other watercourses throughout the Subbasin. The supplementation program differs from other hatchery programs because the fish would be released at different sizes and would return to reproduce naturally in the areas where they are released. The Use of Existing Facilities Alternative proposes using existing production hatcheries and the proposed satellite facilities to meet the need. Facilities at Dworshak National Fish Hatchery, Kooskia National Fish Hatchery, and Hagerman National Fish Hatchery would be used as central incubation and rearing facilities. -

Draft Clearwater Assessment: 8. Fishery Resources
8 Fishery Resources 8.1 Fish Status Currently more than 30 species of fish inhabit the Clearwater subbasin, including 19 native species, two of which have been reintroduced (Table 43). Salmonids and cyprinids are most numerous, representing 10 and 6 species, respectively. Exotic species within the subbasin are generally introduced sport or forage species, and include primarily centrarchids, ictalurids, and salmonids. Five fish species have been chosen as aquatic focal species in this assessment: chinook salmon (Oncorhynchus tshawytscha), steelhead trout (Oncorhynchus mykiss subspecies), westslope cutthroat trout (Oncorhynchus clarki lewisi), bull trout (Salvelinus confluentus) and brook trout (Salvelinus fontinalis). Aquatic focal species may serve as indicators of larger communities, and are listed by federal and/or state agencies as species of concern or, in the case of brook trout, have the potential to negatively impact other selected species. In addition, aquatic focal species had adequate data available for species status, distribution, and habitat use to aid future decision making. Information is also provided for additional species of interest for which only limited data exists, redband trout (Oncorhynchus mykiss subspecies), Pacific lamprey (Lampetra tridentata) and coho salmon (Oncorhynchus kisutch). Although species status is discussed, data limitations for these species prohibits substantial consideration of limiting factors and distribution or condition of existing habitat areas. The resident fishery in Dworshak Reservoir is also considered a substantial fishery resource in the Clearwater subbasin. The Dworshak Reservoir fishery involves multiple species, and is addressed as a single fishery rather than as a large number of individual species. Distribution and status information was compiled for the five aquatic focal species using 23 data sources. -

The Evolution of Inbreeding in Western Redcedar (Thuja Plicata: Cupressaceae)
THE EVOLUTION OF INBREEDING IN WESTERN REDCEDAR (THUJA PLICATA: CUPRESSACEAE) by LISA MARIE O'CONNELL B.A. University of Ottawa, 1993 B.Sc. Dalhousie University, 1995 M.Sc. Queen's University, 1997 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Department of Forest Sciences) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA 2003 © Lisa Marie O'Connell, 2003 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of forfs't Sci e rt c*5 The University of British Columbia Vancouver, Canada Date April H , 2^003 DE-6 (2/88) Abstract Long-lived woody plants usually show high levels of outcrossing, inbreeding depression and genetic diversity compared to other plants. A review of the literature showed a mean oucrossing rate of 83.5 in conifers, and a positive, but weak, correlation between outcrossing and genetic diversity. Among conifers, western redcedar (Thuja plicata, Cupressaceae) has one of the highest rates of self-fertilization and lowest amount of genetic diversity, and thus offers the opportunity to study the evolution of inbreeding in a predominantly outcrossing group of plants. -

Lathyrus Bijugatus
Fire Effects Information System (FEIS) FEIS Home Page Lathyrus bijugatus Table of Contents SUMMARY INTRODUCTION DISTRIBUTION AND OCCURRENCE BOTANICAL AND ECOLOGICAL CHARACTERISTICS FIRE ECOLOGY AND MANAGEMENT OTHER MANAGEMENT CONSIDERATIONS APPENDIX REFERENCES Figure 1—Drypark pea in flower. Photo by Tara Luna, used with permission. SUMMARY This Species Review summarizes the scientific information that was available on drypark pea as of February 2021. Drypark pea is a rare, leguminous forb that occurs in eastern Washington and Oregon, northern Idaho, and northwestern Montana. Within that distribution, it grows in a broad range of biogeoclimatic zones and elevations. As its common name "drypark pea" suggests, it prefers dry soils and open sites. Drypark pea grows in sagebrush-conifer and sagebrush-grassland transition zones; in ponderosa pine, Douglas-fir, and subalpine fir-Engelmann spruce woodlands and forests; and subalpine fir parklands. In conifer communities, it is most common in open stands. Drypark pea has rhizomes that grow out from its taproot. Its roots host nitrogen-fixing Rhizobium bacteria. Drypark pea regenerates from seed and has a soil-stored seed bank; however, information on seed dispersal, viability, and seedling establishment of drypark pea was not available in the literature. Fire probably top-kills drypark pea, and it likely sprouts from its rhizomes and/or caudex after top-kill; however, these responses are undocumented. Only one study provided information on the response of drypark pea to fire. In ponderosa pine forest in northern Idaho, cover and frequency of drypark pea were similar on unburned plots and plots burned under low or high intensity, when 1 averaged across 3 postfire years. -

Characterization of Ecoregions of Idaho
1 0 . C o l u m b i a P l a t e a u 1 3 . C e n t r a l B a s i n a n d R a n g e Ecoregion 10 is an arid grassland and sagebrush steppe that is surrounded by moister, predominantly forested, mountainous ecoregions. It is Ecoregion 13 is internally-drained and composed of north-trending, fault-block ranges and intervening, drier basins. It is vast and includes parts underlain by thick basalt. In the east, where precipitation is greater, deep loess soils have been extensively cultivated for wheat. of Nevada, Utah, California, and Idaho. In Idaho, sagebrush grassland, saltbush–greasewood, mountain brush, and woodland occur; forests are absent unlike in the cooler, wetter, more rugged Ecoregion 19. Grazing is widespread. Cropland is less common than in Ecoregions 12 and 80. Ecoregions of Idaho The unforested hills and plateaus of the Dissected Loess Uplands ecoregion are cut by the canyons of Ecoregion 10l and are disjunct. 10f Pure grasslands dominate lower elevations. Mountain brush grows on higher, moister sites. Grazing and farming have eliminated The arid Shadscale-Dominated Saline Basins ecoregion is nearly flat, internally-drained, and has light-colored alkaline soils that are Ecoregions denote areas of general similarity in ecosystems and in the type, quality, and America into 15 ecological regions. Level II divides the continent into 52 regions Literature Cited: much of the original plant cover. Nevertheless, Ecoregion 10f is not as suited to farming as Ecoregions 10h and 10j because it has thinner soils. -

Softwood Insect Pests
Forest & Shade Tree Insect & Disease Conditions for Maine A Summary of the 2011 Situation Forest Health & Monitoring Division Maine Forest Service Summary Report No. 23 MAINE DEPARTMENT OF CONSERVATION March 2012 Augusta, Maine Forest Insect & Disease—Advice and Technical Assistance Maine Department of Conservation, Maine Forest Service Insect and Disease Laboratory 168 State House Station, 50 Hospital Street, Augusta, Maine 04333-0168 phone (207) 287-2431 fax (207) 287-2432 http://www.maine.gov/doc/mfs/idmhome.htm The Maine Forest Service/Forest Health and Monitoring (FH&M) Division maintains a diagnostic laboratory staffed with forest entomologists and a forest pathologist. The staff can provide practical information on a wide variety of forest and shade tree problems for Maine residents. Our technical reference library and insect collection enables the staff to accurately identify most causal agents. Our website is a portal to not only our material and notices of current forest pest issues but also provides links to other resources. A stock of information sheets and brochures is available on many of the more common insect and disease problems. We can also provide you with a variety of useful publications on topics related to forest insects and diseases. Submitting Samples - Samples brought or sent in for diagnosis should be accompanied by as much information as possible including: host plant, type of damage (i.e., canker, defoliation, wilting, wood borer, etc.), date, location, and site description along with your name, mailing address and day-time telephone number or e-mail address. Forms are available (on our Web site and on the following page) for this purpose. -
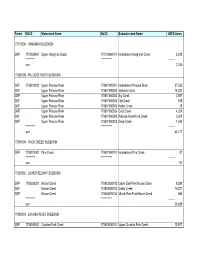
Forest HUC5 Watershed Name HUC6 Subwatershed Name USFS Acres
Forest HUC5 Watershed Name HUC6 Subwatershed Name USFS Acres 17010306 - HANGMAN SUBBASIN CNF 1701030601 Upper Hangman Creek 170103060101 Headwaters Hangman Creek 2,245 ********** ************ --------- sum 2,245 17060108 - PALOUSE RIVER SUBBASIN CNF 1706010803 Upper Palouse River 170601080301 Headwaters Palouse River 27,352 CNF Upper Palouse River 170601080302 Meadow Creek 14,237 CNF Upper Palouse River 170601080303 Big Creek 2,857 CNF Upper Palouse River 170601080304 Flat Creek 839 CNF Upper Palouse River 170601080305 Hatter Creek 16 CNF Upper Palouse River 170601080306 Gold Creek 4,224 CNF Upper Palouse River 170601080308 Palouse River/Rock Creek 3,300 CNF Upper Palouse River 170601080309 Deep Creek 1,346 ********** ************ --------- sum 54,171 17060109 - ROCK CREEK SUBBASIN CNF 1706010901 Pine Creek 170601090101 Headwaters Pine Creek 87 ********** ************ --------- sum 87 17060302 - LOWER SELWAY SUBBASIN CNF 1706030201 Moose Creek 170603020102 Upper East Fork Moose Creek 8,290 CNF Moose Creek 170603020103 Cedar Creek 16,271 CNF Moose Creek 170603020104 Middle East Fork Moose Creek 686 ********** ************ --------- sum 25,639 17060303 - LOCHSA RIVER SUBBASIN CNF 1706030301 Crooked Fork Creek 170603030101 Upper Crooked Fork Creek 18,907 Forest HUC5 Watershed Name HUC6 Subwatershed Name USFS Acres CNF Crooked Fork Creek 170603030102 Boulder Creek 15,627 CNF Crooked Fork Creek 170603030103 Lower Crooked Fork Creek 11,766 CNF Crooked Fork Creek 170603030104 Upper Brushy Fork Creek 5,142 CNF Crooked Fork Creek 170603030105 Spruce -

Wolverines in Idaho 2014–2019
Management Plan for the Conservation of Wolverines in Idaho 2014–2019 Prepared by IDAHO DEPARTMENT OF FISH AND GAME July 2014 2 Idaho Department of Fish & Game Recommended Citation: Idaho Department of Fish and Game. 2014. Management plan for the conservation of wolverines in Idaho. Idaho Department of Fish and Game, Boise, USA. Idaho Department of Fish and Game – Wolverine Planning Team: Becky Abel – Regional Wildlife Diversity Biologist, Southeast Region Bryan Aber – Regional Wildlife Biologist, Upper Snake Region Scott Bergen PhD – Senior Wildlife Research Biologist, Statewide, Pocatello William Bosworth – Regional Wildlife Biologist, Southwest Region Rob Cavallaro – Regional Wildlife Diversity Biologist, Upper Snake Region Rita D Dixon PhD – State Wildlife Action Plan Coordinator, Headquarters Diane Evans Mack – Regional Wildlife Diversity Biologist, McCall Subregion Sonya J Knetter – Wildlife Diversity Program GIS Analyst, Headquarters Zach Lockyer – Regional Wildlife Biologist, Southeast Region Michael Lucid – Regional Wildlife Diversity Biologist, Panhandle Region Joel Sauder PhD – Regional Wildlife Diversity Biologist, Clearwater Region Ben Studer – Web and Digital Communications Lead, Headquarters Leona K Svancara PhD – Spatial Ecology Program Lead, Headquarters Beth Waterbury – Team Leader & Regional Wildlife Diversity Biologist, Salmon Region Craig White PhD – Regional Wildlife Manager, Southwest Region Ross Winton – Regional Wildlife Diversity Biologist, Magic Valley Region Additional copies: Additional copies can be downloaded from the Idaho Department of Fish and Game website at fishandgame.idaho.gov/wolverine-conservation-plan Front Cover Photo: Composite photo: Wolverine photo by AYImages; background photo of the Beaverhead Mountains, Lemhi County, Idaho by Rob Spence, Greater Yellowstone Wolverine Program, Wildlife conservation Society. Back Cover Photo: Release of Wolverine F4, a study animal from the Central Idaho Winter Recreation/Wolverine Project, from a live trap north of McCall, 2011. -

Preliminary Classification of Leotiomycetes
Mycosphere 10(1): 310–489 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/7 Preliminary classification of Leotiomycetes Ekanayaka AH1,2, Hyde KD1,2, Gentekaki E2,3, McKenzie EHC4, Zhao Q1,*, Bulgakov TS5, Camporesi E6,7 1Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, 57100, Thailand 3School of Science, Mae Fah Luang University, Chiang Rai, 57100, Thailand 4Landcare Research Manaaki Whenua, Private Bag 92170, Auckland, New Zealand 5Russian Research Institute of Floriculture and Subtropical Crops, 2/28 Yana Fabritsiusa Street, Sochi 354002, Krasnodar region, Russia 6A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy. 7A.M.B. Circolo Micologico “Giovanni Carini”, C.P. 314 Brescia, Italy. Ekanayaka AH, Hyde KD, Gentekaki E, McKenzie EHC, Zhao Q, Bulgakov TS, Camporesi E 2019 – Preliminary classification of Leotiomycetes. Mycosphere 10(1), 310–489, Doi 10.5943/mycosphere/10/1/7 Abstract Leotiomycetes is regarded as the inoperculate class of discomycetes within the phylum Ascomycota. Taxa are mainly characterized by asci with a simple pore blueing in Melzer’s reagent, although some taxa have lost this character. The monophyly of this class has been verified in several recent molecular studies. However, circumscription of the orders, families and generic level delimitation are still unsettled. This paper provides a modified backbone tree for the class Leotiomycetes based on phylogenetic analysis of combined ITS, LSU, SSU, TEF, and RPB2 loci. In the phylogenetic analysis, Leotiomycetes separates into 19 clades, which can be recognized as orders and order-level clades. -

Potlatch River Steelhead Monitoring and Evaluation Project
POTLATCH RIVER STEELHEAD MONITORING AND EVALUATION PROJECT 2017 AND 2018 BIENNIAL REPORT Prepared by: Brian A. Knoth, Fisheries Biologist Brett J. Bowersox, Fishery Staff Biologist Jason T. Fortier, Fisheries Technician 2 IDFG Report Number 20-15 March 2021 POTLATCH RIVER STEELHEAD MONITORING AND EVALUATION PROJECT 2017 AND 2018 BIENNIAL REPORT By: Brian A. Knoth Brett J. Bowersox Jason T. Fortier Idaho Department of Fish and Game 600 South Walnut Street P.O. Box 25 Boise, ID 83707 IDFG Report Number 20-15 March 2021 TABLE OF CONTENTS Page ABBREVIATIONS AND ACRONYMS .........................................................................................1 FOREWORD ..............................................................................................................................2 PROJECT OVERVIEW ...............................................................................................................2 PROJECT DESIGN AND OBJECTIVES ..................................................................................... 3 PROJECT TIMELINE ..................................................................................................................4 REPORT STRUCTURE ..............................................................................................................4 LITERATURE CITED ...............................................................................................................5 FIGURES ....................................................................................................................................7