Title Ecological Studies on Dispersal Flight and Host Selection of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MYCOTAXON Volume 104, Pp
MYCOTAXON Volume 104, pp. 399–404 April–June 2008 Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae T. C. Harrington1*, S. W. Fraedrich2 & D. N. Aghayeva3 *[email protected] 1Department of Plant Pathology, Iowa State University 351 Bessey Hall, Ames, IA 50011, USA 2Southern Research Station, USDA Forest Service Athens, GA 30602, USA 3Azerbaijan National Academy of Sciences Patamdar 40, Baku AZ1073, Azerbaijan Abstract — An undescribed species of Raffaelea earlier was shown to be the cause of a vascular wilt disease known as laurel wilt, a severe disease on redbay (Persea borbonia) and other members of the Lauraceae in the Atlantic coastal plains of the southeastern USA. The pathogen is likely native to Asia and probably was introduced to the USA in the mycangia of the exotic redbay ambrosia beetle, Xyleborus glabratus. Analyses of rDNA sequences indicate that the pathogen is most closely related to other ambrosia beetle symbionts in the monophyletic genus Raffaelea in the Ophiostomatales. The asexual genus Raffaelea includes Ophiostoma-like symbionts of xylem-feeding ambrosia beetles, and the laurel wilt pathogen is named R. lauricola sp. nov. Key words — Ambrosiella, Coleoptera, Scolytidae Introduction A new vascular wilt pathogen has caused substantial mortality of redbay [Persea borbonia (L.) Spreng.] and other members of the Lauraceae in the coastal plains of South Carolina, Georgia, and northeastern Florida since 2003 (Fraedrich et al. 2008). The fungus apparently was introduced to the Savannah, Georgia, area on solid wood packing material along with the exotic redbay ambrosia beetle, Xyleborus glabratus Eichhoff (Coleoptera: Curculionidae: Scolytinae), a native of southern Asia (Fraedrich et al. -

Appl. Entomol. Zool. 41 (1): 123–128 (2006)
Appl. Entomol. Zool. 41 (1): 123–128 (2006) http://odokon.ac.affrc.go.jp/ Death of Quercus crispula by inoculation with adult Platypus quercivorus (Coleoptera: Platypodidae) Haruo KINUURA1,* and Masahide KOBAYASHI2 1 Kansai Research Center, Forestry and Forest Products Research Institute; Kyoto 612–0855, Japan 2 Kyoto Prefectural Forestry Experimental Station; Kyoto 629–1121, Japan (Received 10 December 2004; Accepted 27 October 2005) Abstract Adult Platypus quercivorus beetles were artificially inoculated into Japanese oak trees (Quercus crispula). Two inocu- lation methods were used: uniform inoculation through pipette tips, and random inoculation by release into netting. Four of the five trees that were inoculated uniformly died, as did all five trees that were inoculated at random. Seven of the nine dead trees showed the same wilting symptoms seen in the current mass mortality of oak trees. Raffaelea quercivora, which has been confirmed to be the pathogenic fungus that causes wilt disease and is usually isolated from the mycangia of P. quercivorus, was isolated from all of the inoculated dying trees. Trees that died faster showed a higher density of beetle galleries that succeeded in producing offspring. We found positive relationships between the density of beetle galleries that succeeded in producing offspring and the rate of discoloration in the sapwood and the isolation rate of R. quercivora. Therefore, we clearly demonstrated that P. quercivorus is a vector of R. quercivora, and that the mass mortality of Japanese oak trees is caused by mass attacks of P. quercivorus. Key words: Mass mortality; oak; pathogenic fungi; Raffaelea quercivora; vector An inoculation test on some smaller trees in the INTRODUCTION nursery with this fungus, which was later described In Japan, the mass mortality of oak trees (Quer- as Raffaelea quercivora Kubono et Shin. -

Biology and Ecology of Leptographium Species and Their Vectos As Components of Loblolly Pine Decline Lori G
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline Lori G. Eckhardt Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Plant Sciences Commons Recommended Citation Eckhardt, Lori G., "Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline" (2003). LSU Doctoral Dissertations. 2133. https://digitalcommons.lsu.edu/gradschool_dissertations/2133 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. BIOLOGY AND ECOLOGY OF LEPTOGRAPHIUM SPECIES AND THEIR VECTORS AS COMPONENTS OF LOBLOLLY PINE DECLINE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Plant Pathology & Crop Physiology by Lori G. Eckhardt B.S., University of Maryland, 1997 August 2003 © Copyright 2003 Lori G. Eckhardt All rights reserved ii ACKNOWLEDGMENTS I gratefully acknowledge the invaluable input provided by my dissertation advisor, Dr. John P. Jones. Among many other things, he has demonstrated his patients, enthusiasm and understanding as I struggled to pursue my graduate studies. I am indebted to Dr. Marc A. Cohn, for his guidance, encouragement, support and most of all, his friendship. -

Screening Aid Platypus Quercivorus (Murayama), Megaplatypus Mutatus (Chapuis)
Ambrosia Beetles Screening Aid Platypus quercivorus (Murayama), Megaplatypus mutatus (Chapuis) Joseph Benzel 1) Identification Technology Program (ITP) / Colorado State University, USDA-APHIS-PPQ-Science & Technology (S&T), 2301 Research Boulevard, Suite 108, Fort Collins, Colorado 80526 U.S.A. (Email: [email protected]) This CAPS (Cooperative Agricultural Pest Survey) screening aid produced for and distributed by: Version 5 USDA-APHIS-PPQ National Identification Services (NIS) 30 June 2015 This and other identification resources are available at: http://caps.ceris.purdue.edu/taxonomic_services The ambrosia beetles (Platypodinae) (Fig. 1) are an enigmatic group of bark beetles native to the tropical regions of the world. Within this group two species are considered potentially invasive pests: Platypus quercivorus (Murayama) and Megaplatypus mutatus (Chapuis). Platypus quercivorus feeds primarily on oaks (Quercus) and other broad leaf trees and spreads the ambrosia fungus Raffaelea quercivora which may be the agent of Japanese oak disease. Megaplatypus mutatus primarily attacks walnut (Juglans), poplar (Populus), and apple (Malus) trees but will infest a variety of other broadleaf trees. It does little direct damage to the tree, but its boring can reduce the tree’s structural integrity and discoloration caused by symbiotic fungi reduce wood quality (Figs. 2-3). Platypodinae is currently considered a subfamily of Curculionidae which Fig. 1: Platypus sp. on tree (photo by, is comprised of weevils and bark beetles. Members of this family are Andrea Battisti, Universita di Padova, highly variable but almost all species share a distinct club on the end Bugwood.org). of their antennae consisting of three segments. In general, members of Platypodinae are small (<10mm long) elongate beetles of a reddish brown or black color. -

Invasive Forest Pests – What Risk to the UK?
Invasive forest pests – what risk to the UK? Daegan Inward Invasive species can be very economically and environmentally damaging: - Predate, compete or hybridise with native species - Influence loss of native species & ecosystem services - Most serious pests and diseases of trees and forests are non-native Invasive tree pathogens in the UK: Dothistroma needle blight Ash dieback Dutch elm disease Phytophthora ramorum 2 21/04/2020 Some ‘high profile’ invasive forest pests in the UK: Horse chestnut leaf miner 5mm Oak processionary moth 3 21/04/2020 Asian Longhorn beetle (ALB) •ALB is native to E. Asia, and feeds within a variety of broadleaf trees. It has become a pest across Europe and N. America • In 2009 a live adult ALB was found by a local resident at Paddock Wood, Kent. • Inspection of the area located a suspected source: a premises importing stone from China. 4 21/04/2020 ALB Eradication actions, 2013 -Initial rapid ground survey; felling & detailed inspection. -All potential host trees within 100m of an infested tree felled, inspected & destroyed on-site. -All broad-leaf trees within 500m radius inspected from ground, repeated over next 3-4yrs. Final Tally: 2166 trees felled (incl. 627 from private gardens) 66 infested trees (10 different tree species) ALB declared eradicated in 2018 – the ecology of the species made it possible in this case (extended life cycle in UK) 5 21/04/2020 Scolytinae: Bark & ambrosia beetles Majority are ‘decomposers’. Include some of most significant temperate forest pests Some with capacity to kill mature trees Key biosecurity threat, easily transported Increased risk under CC due to increased frequency of drought stress US has 58 exotic Scolytinae established Europe has >20 established Scolytinae Until recently, the UK recorded only 1 (Dendroctonus micans) > 30% of Coleoptera interceptions in the UK are Scolytinae 6 21/04/2020 Mechanisms of spread Eric Allen, Canada 7 21/04/2020 Bark vs ambrosia beetles Most Scolytinae have a symbiotic relationship with fungi; may be actively or passively vectored. -

R. L. Meagher, Jr.R and J. C. Kgaspi 2 Texas A&M University Agricultural
S O U T IIW E S T E R N E N T O M O L O G IS T N IA R .2003 M ttIIN ‐F IE L D D IST R IB U T IO N O F T H R E E H O M O PT E R A N SP E C ttS IN T E X A S SU G A R C A N E R. L. Meagher,Jr.r andJ. C. kgaspi 2 TexasA&M University Agricultural Researchand ExtensionCenter 2415EastHighway 83 Weslaco,Texas 78596 ABSTRACT Sugarcanefields composed ofeither'CP 70-321'or'NCo 310'weresampled during 1993 and 1994 for three speciesofhomopteran insects. The West Indian canefly, Saccharosydne saccharivora(tlestwood), wasthe mostabundant speiies collectedwith densitiesreaching over 40 per shoot. Populationdensities varied sigrificantly throughoutthe seasonin 1993but were similar in 1994. No differencein densitieswere found between sugarcane cultivars. Both the sugarcanedelphacid, Perhinsiella saccharicidaKirkaldyand the leafhopperDraeculacephala portola Ball, were found in low numbers(< 1.0per shoot). P. saccharicidareached its highest levelslater in the season,and 'CP 70-321'shoots harbored more individuals than 'NCo 310' shoots.Although D. portola dertsitieswere low, differe,ncesamong fields andbetwecn cultivars were found. Aggregation,as measuredusing Taylor a and b coefficients, was shown to be greaterfor ,S.saccharivora than the other species. INTRODUCTION Sugarcane(interspecific hybrids of Saccharum)has been commercially grown in a tlree- county areaof southemTexas since 1972. Stemboringpyralids, Mexican rice borer, Eoreuma loftini (Dyar), andsugarcane borer, Diatraea saccharalis(F.), havebeen the most seriousinsect pests of the in{ustry (Meagher et al. 1994); however, other insects are active in the Texas sugarcaneagroecos)Nstem. -

Conifer Bole Utilization by Wood-Boring Beetles in Western Oregon Cfatc75
CfAtc75-;,) ?-437`,, ) Conifer bole utilization by wood-boring beetles in western Oregon /p i H. ZHONG AND T. D. SCHOWALTER1 Department of Entomology, Oregon State University, Corvallis, OR 97331, U.S.A. Received December 7, 1988 Accepted April 4, 1989 ZHONG, H., and SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19: 943-947. We studied wood excavation by scolytid and cerambycid beetles in decomposing boles of four conifer species during the first two years on the ground in western Oregon. Colonization density and gallery volumes were measured in experi- mental boles (0.5 m diameter x 5 m length) of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), western hemlock (Tsuga heterophylla (Raf.) Sarg.), Pacific silver fir (Abies amabilis (Dougl.) Forbes), and western red cedar ( Thuja plicata Donn). Ambrosia beetles (Coleoptera: Scolytidae) colonized boles only during the 1st year and were essentially restricted to Douglas-fir and western hemlock (removing 0.2% of the sapwood volume). Bark beetles (Coleoptera: Scolytidae) colonized boles only in the 1st year, primarily in Douglas-fir and Pacific silver fir (removing 7-8% of the phloem surface area). Wood borers (Coleoptera: Cerambycidae) excavated an additional 2.3% of the phloem surface area of Pacific silver fir in the 1st year and continued to excavate all species except Douglas-fir during the 2nd year. Consequences for the decomposition process are discussed. ZHONG, H., et SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19 : 943-947. Lexcavation du bois par les coleopteres dans les troncs en decomposition de quatre especes de coniferes a ete etudiee durant les 2 premieres annees de leur position au sol dans louest de ]Oregon. -

Raffaelea Quercivora
Raffaelea quercivora Scientific Name Raffaelea quercivora Kubono and Shin. Ito, 2002 Synonyms: none known Common Names Japanese oak wilt JOW Japanese oak disease fungus Type of Pest Fungus Figure 1. Raffaelea quercivora infestation in an oak forest. Troy Kimoto, CFIA, Bugwood.org. Taxonomic Position Class: Sordariomycetes Order: Ophiostomatales Family: Ophiostomataceae Reason for inclusion in manual CAPS Pest of Economic and Environmental Importance CAPS Oak commodity survey list Pest Description On the outside of host trees, there is little visible sign of Raffaelea quercivora. Symptoms of leaf discoloration and wilting are visible on infected hosts (Kuroda, 2001), but these may be caused by other wilt pathogens. Raffaelea quercivora can be seen in cross-sections of symptomatic trees as a brown to black discoloration and small white pustules originating by the tunnels formed by its vector, Platypus quercivorus (Fig. 2) (Batra, 1967a). Figure 2. Sporodochia (small white pustules) growing on a cross section of a maple. Reproduced from Batra (1967a) with permission from Mycologia. ©The Mycological Society of America. 1 Last Update: September 28, 2018 Identification of R. quercivora requires isolation of the pathogen from host material and growth in culture for morphological identification. Colonies produced on potato dextrose agar (PDA) at 20-25°C (68-77°F) spread rapidly and can reach 80 mm (~3 1/8 in) diameter in five days. The colony itself initially has a white, water- soaked appearance that shifts to a pale olive to brown-olive color after two weeks, taking on a Figure 3: Left: Raffaelea quercivora hyphae invading fragrant odor reminiscent of ethyl living host cells (radial section). -
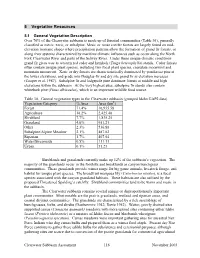
Draft Clearwater Assessment: 5. Vegetative Resources
5 Vegetative Resources 5.1 General Vegetation Description Over 70% of the Clearwater subbasin is made up of forested communities (Table 30), generally classified as mesic, xeric, or subalpine. Mesic or moist conifer forests are largely found on mid- elevation montane slopes where precipitation patterns allow the formation of grand fir forests, or along river systems characterized by maritime climatic influences such as occur along the North Fork Clearwater River and parts of the Selway River. Under these unique climatic conditions grand fir gives way to western red cedar and hemlock (Tsuga heterophylla) stands. Cedar forests often contain unique plant species, including two focal plant species, crenulate moonwort and mountain moonwort. Xeric or dry forests are characteristically dominated by ponderosa pine at the lower elevations, and grade into Douglas-fir and dry site grand fir as elevation increases (Cooper et al. 1987). Subalpine fir and lodgepole pine dominate forests at middle and high elevations within the subbasin. At the very highest sites, subalpine fir stands also contain whitebark pine (Pinus albicaulus), which is an important wildlife food source. Table 30. General vegetation types in the Clearwater subbasin (grouped Idaho GAP2 data) Vegetation Category % Area Area (km2) Forest 71.4% 16,955.58 Agriculture 10.2% 2,425.48 Shrubland 7.7% 1,835.25 Grassland 4.0% 951.21 Other 2.3% 536.88 Subalpine/Alpine Meadow 2.1% 487.02 Riparian 1.7% 407.04 Water/Streamside 0.5% 111.11 Urban 0.1% 31.23 Shrublands and grasslands currently make up 12% of the subbasin’s vegetation. The majority of the grasslands occur in the foothills and breaklands as canyon bunchgrass communities. -

Oakambrosiabeetle.Pdf
1 The Oak Ambrosia Beetle, Platypus quercivorus, belongs to the wood boring ambrosia beetle Family Platypodidae (2). Platypodidae is a very diverse family with over 1,000 species distributed primarily throughout the tropics (1). Only seven species, all belonging to the genus Platypus, are found in the United States of which four occur in Florida (1). Oak Ambrosia Beetle was first described from specimens in Taiwan in 1925 (4). This beetle is part of a ambrosia beetle-fungus complex, serving as a vector of Japanese Oak Wilt, Raffaelea quercivora , a fungal disease that is devastating several species of oaks in Japan(6). 2 The global distribution of the Oak Ambrosia Beetle is limited to the temperate and subtropical forests of Asia and parts of Oceania (3). The pest is widespread throughout Japan. However, populations are also present in Bengal (India), Java (Indonesia), Papua New Guinea, and Taiwan (2). 3 This host map created by USDA-APHIS-PPQ-CPHST in the 2011 Exotic Wood Borer and Bark Beetle National Survey Manual illustrates the potential risk of Oak Ambrosia Beetle distribution based on the density of host acreage in different counties (11). Note the higher relative risk along the Atlantic seaboard and southeastern US. USDA-APHIS-PPQ-CPHST and NCSU cooperators are working to revise the NCSU/APHIS Plant Pest Forecast System, a tool which will be able to combine available distribution and survey data for the purpose of predicting the potential distribution of pests and diseases in new areas. CPHST is also revising the Exotic Wood Borer and Bark Beetle National Survey Manual as part of the Cooperative Agricultural Pest Survey (CAPS) Program. -

Effects of Pathogens and Bark Beetles on Forests
Utah State University DigitalCommons@USU Quinney Natural Resources Research Library, The Bark Beetles, Fuels, and Fire Bibliography S.J. and Jessie E. 1993 Effects of Pathogens and Bark Beetles on Forests D J. Goheen E M. Hansen Follow this and additional works at: https://digitalcommons.usu.edu/barkbeetles Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Forest Biology Commons, Forest Management Commons, and the Wood Science and Pulp, Paper Technology Commons Recommended Citation Goheen, D. and Hansen, E. Effects of pathogens and bark beetles on forests, pp. 175-196 in: TD Schowalter & GM Filip (eds) Beetle-Pathogen Interactions in Conifer Forests, Academic Press, London. This Contribution to Book is brought to you for free and open access by the Quinney Natural Resources Research Library, S.J. and Jessie E. at DigitalCommons@USU. It has been accepted for inclusion in The Bark Beetles, Fuels, and Fire Bibliography by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. .... 9.... Effects of Pathogens and Bark Beetles on Forests D. J. GOHEEN1 and E. M. HANSEN2 1USDA Forest Service, Pacific Northwest Region, Portland, OR, USA 2Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, USA 9.1 Introduction 175 9.2 Effects of Bark Beetle-Pathogen Interactions 176 9.2.1 Interactions in Pseudotsuga Forests: Phellinus and Deruiroctonus 176 9.2.2 Interactions in Pseudotsuga Forests: Leptographium and Bark Beetles 180 9.2.3 Interactions in Pinus ponderosa Forests 183 9.2.4 Interactions in Pinus contorta Forests 186 9.2.5 Interactions in Abies Forests 187 9.2.6 Interactions in Eastern Forests 190 9.3 Conclusions 191 References 191 9.1 INTRODUCTION Pathogenic fungi and bark beetles are important components of most coniferous forest ecosystems. -

Oak Ambrosia Beetle
Oak Ambrosia Beetle Platypus quercivorus (Coleoptera: Platypodidae) Phenology/Degree-Day and Climate Suitability Model White Paper for USPEST.ORG Prepared for USDA APHIS PPQ Version 1.0. 3/27/2020 Brittany Barker and Len Coop Department of Horticulture and Oregon IPM Center Oregon State University Summary A phenology model and temperature-based climate suitability model for the oak ambrosia beetle (OAB), Platypus quercivorus (Murayama), was developed using data from available literature and through modeling in CLIMEX v. 4 (Hearne Scientific Software, Melbourne, Australia; Kriticos et al. 2016), Maxent (Phillips et al. 2006), and DDRP (Degree-Days, Risk, and Pest event mapping; under development for uspest.org). Introduction Platypus quercivorus is the vector of a pathogenic fungus (Raffaelea quercivora) that causes Japanese oak wilt disease, which has caused massive mortality in oak trees (Quercus spp.) in Japan and can infect other species in the Fagaceae family including chestnut (Castanea spp.), chinquapin (Castanopsis spp.), and stone oaks (Lithocarpus spp.). Wood boring by P. quercivorus can slow growth and increase mortality of both host and adjacent non-host trees, and it predisposes trees to further damage by secondary pests (Davis et al. 2005). At least 28 species of Quercus in the continental U.S. may potentially serve as hosts (Davis et al. 2005). The establishment of P. quercivorus in the U.S. would likely have high environmental and economic impacts because oak forests provide numerous ecosystem services including high value timber for industry, wildlife habitat, and valued environments for recreation and other cultural activities. Phenology model Objective.—We assessed the literature to derive developmental thresholds and life stage durations for P.