Isolation, Molecular Characterization and Sero-Prevalence Study of Foot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Current Status of Ectoparasites in Sheep and Management Practices
ary Scien in ce r te & e T V e f c h o n n l Journal of VVeterinaryeterinary Science & Bedada et al., J Veterinar Sci Technolo 2015, S:10 o o a a l l n n o o r r g g u u y y DOI: 10.4172/2157-7579.S10-002 o o J J ISSN: 2157-7579 TTechnologyechnology Research Article Open Access Current Status of Ectoparasites in Sheep and Management Practices against the Problem in Ectoparasites Controlled and Uncontrolled Areas of Arsi Zone in Oromia Region, Ethiopia Hailegebriel Bedada, Getachew Terefe and Yacob Hailu Tolossa* College of Veterinary Medicine and Agriculture, Addis Ababa University, Debre Zeit-34, Ethiopia Abstract A cross sectional study was conducted to determine the prevalence of ectoparasites of sheep in ectoparasites controlled and uncontrolled areas, assess major risk factors and evaluate effects of ectoparasites on livelihood of farmer in ectoparasites controlled and uncontrolled areas of Arsi zone. A total of 969 sheep (646 sheep from controlled and 323 sheep from uncontrolled areas) were examined for ectoparasites. From controlled 371 (57.43%) and from uncontrolled area 285 (88.24%) were found to be infested with ectoparasites. The ectoparasites identified in controlled area were B. ovis 48.9%, Linognathus spp 0.93%, sheep keds 7.4%, 2.32% B. decoloratus, 1.46% A. variegatum, 1.08% A. gemma, 4.59% R. evertsi evertsi, and 0.31% mixed ticks infestation and 12.5% mixed infestation with various ectoparasites. Similarly from uncontrolled area identified B. ovis 81.4%, Linognathus spp 0.9%, 1.79% B. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

Religious Sformation Among the Arsi Oromo of Ethiopia Gemechu J. Geda
PILGRIMAGES AND SYNCRETISM: RELIGIOUS TRANSFORMATION AMONG THE ARSI OROMO OF ETHIOPIA GEMECHU J. GEDA A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN RELIGIOUS STUDIES FACULTY O F CULTURAL STUDIES UNIVERSITY OF BAYREUTH GERMANY 23 SEPTEMBER 2013 SUMMARY Currently, the majority of the Arsi Oromo are either Muslims or Christians. However, most of them still practice their traditional beliefs passed down through generations by their forefathers, such as Waaqeffannaa , and attend various rituals related to it . Waaqeffannaa is a religion based on belief in one God known to the Oromo as Waaqa , which according to the Oromo is the creator of the entire universe. The Oromo belief of the existence of Waaqa is based on observing what they call his works, such as the presence of various seasons, rain, sun, darkness, growing of crops, existence of water bodies, mountains, trees and other living things. Contrary to Christianity, Islam, and other religions, Waaqeffannaa does not require the construction of religious houses for the veneration of Waaqa or for thanking him for his good deeds. Instead, the Oromo who are followers of Waaqeffannaa thank Waaqa by travelling to natural physical bodies such as rivers, lakes, forests, and mountains, which they believe are created by Waaqa himself. Waaqeffannaa is believed to be a free will religion, where a believer does not need to calculate in order to obtain certain advantages, such as going to heaven in the afterlife for adhering to Waaqa . To the same effect, a believer would not face some kind of punishment for abandoning Waaqa . -
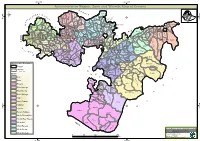
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

1. Program Description
FSCP-GIAF-ETHIOPIA GRANT APPLICATION GUIDE 1. Program Description Cultivating New Frontiers in Agriculture (CNFA) is implementing the 34-month Farm Service Center Program (FSCP) in partnership with the GIZ Green Innovation Centres for the Agriculture and Food Sector in Ethiopia (GIAF-Ethiopia). The project will establish five private retail input supply stores known as Farm Service Centers (FSCs). These Ethiopian-owned and operated enterprises are designed to improve the productivity, food security and incomes of smallholder famers in the Arsi zone of Ethiopia by linking them to high-quality agricultural inputs and extension services. The project is strategically aligned to build the role of the private sector and is connected to CNFA’s other initiatives in Ethiopia, which also work in the development of FSCs. In addition, and in line with the larger GIAF-Ethiopia initiatives the project will establish FSCs in the following five woredas recommended by the Federal Ministry of Agriculture and Natural Resources and the Oromia Bureau of Agriculture - Hitosa, Lude-Hitosa, Arsi-Robe, Digelu-Tijo and Tiyo. The project will also focus on up-scaling of innovations and best practices for wheat and faba bean production. FSCP-GIAF-Ethiopia is a follow-on to the successful Commercial Farm Service Program, which piloted CNFA’s Farm Service Center (FSC) Model in Ethiopia for the first time. CNFA’s Farm Service Center Model is a market-based, private sector model that applies matching grants and training methodology to establish small and medium-sized enterprises (SMEs) that deliver farm supplies and services. FSCs are located in larger townships and serve as rural development centers that meet the needs of private farmers in their communities. -

OROMIA REGION : Who Does What Where (3W) - WASH Sector (As of 24 June 2013)
(as of 24 June 2013) OROMIA REGION : Who Does What Where (3W) - WASH Sector Tigray Beneshangul Amhara Afar Gumu Afar Amhara Intermon Oxfam: HEKS: CPAR: WVI: Beneshangul Gumu k k k k Dire Dawa Addis Ababa Hareri Intermon Oxfam: Plan Int.: CARE: Gambela Oromia WVE: WVI: Mercy Corps: Somali k ! Wara k k k SNNPR Save the ! CISP: Jarso k ! Childern:k Ababo Horo Abay WVI: Chomen ! CARE: CRS: Guduru ! Dire Dawa ! ! Yaya k k ! Nejo Gulele ! WVI: WVI: Jimma CRS: Meta North ! ! k k Guduru k Goro ! Babo Genete Robi Shewa(R4) Meta ! ! ! Gutu Kersa West ! Gursum ! Jeldu ! ! Wellega TearFund: ! Deder Lalo ! West k Tulo Harari Mieso Asabi East Shewa Sibu Ambo Malka! Wellega Zuria Sire Dendi Addis Ababa Balo ! CRS, WVI ! WVI: k Kelem ! WVI: k Gemechis ! k ! Babile ! Midega CRS: Wellega Save the ! WVI: Habro ! ! West South k Tola ! Wenchi ! Kersana Childern:k ! East Boset Mercy Corps: West Tole Harerge East k Malima Shewa Shewa ! Daro Harerge ZOA: Adama ! Lebu Jeju Boke ! B!ora Guna Gechi HEKS: ! GOAL: Ilubabor ! k k ! Mercy Corps: Dugda! CRS, Hawi WVI: ! k Sekoru Gudina k HEKS k ! ADRA, HEKS ! k Diksis Plan Int.: CISP: Arsi k k Gambela Adami ! Kersa WVI: Tulu Jido ! Dorcas Aid Int.: k ! CRS: IRC: k Kombolcha Degeluna Seru k Jimma ! Tijo Omo Nada GOAL: ! Arsi LVIA: k ! Negele Agencies' locations and ! Save the Shalla Gasera Seweyna area of interventions are Agarfa Children:k GOAL, LIVA, HEKS: ! ! Shashemene Kore ! ! depicted based on the Save the Children: Zuria ! Sinana Siraro Dinsho ! Ginir recent available information. ! ! WVI: Kofele West ! Adaba -

(Tor) Assessment of Production, Economic and Social Impact of Interventions with Innovations on Selected Fa
Terms of Reference (ToR) Assessment of production, economic and social impact of interventions with Innovations on selected farms with wheat and faba bean production in Arsi 1. Background Ethiopia has very diverse bio-physical environment with a variety of ecosystems, with significant differences in climate, soil properties, vegetation types, agricultural potential, biodiversity and water resources. That results in different farming systems and farming products. The crop subsector which, on average, accounted for about 30 percent of GDP has been the major contributor to the growth of the agricultural value added during the period of the GTP (Growth and Transformation Plan). The productivity of major food crops (Cereals, Pulses, and Oil seeds) reached an annual average level of 17.6 quintal (100 kg) per hectare. Further increase in production and productivity of major crops will continue to be a priority in the next five years (2016-2020) by fully implementing strategies such as delivering effective extension services, supplying agricultural inputs and conducting famers training. The participation of several actors like international partners and private business will be encouraged and supported as well by the government. In general, during the period of the second Growth and Transformation Plan, objectives are broadly set in terms of increasing crop and livestock production and productivity, promoting natural resource conservation and utilization, ensuring food security and disaster prevention and preparedness. In this regard, the German -

Xerox University Microfilms
INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spiiced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

ETHIOPIA Food Security Outlook January to June 2011
ETHIOPIA Food Security Outlook January to June 2011 Following the meher harvest, which began in October Figure 1. Current estimated food security outcomes, 2010, food security has generally improved in the January 2011 meher producing parts of the country. However, due to crop damage caused by widespread floods and other weather related shocks the meher harvest is likely to be lower than initially anticipated. The Humanitarian Requirement Documents outlining assistance needs is expected to be released in February 2011. Although the National Meteorology Agency has not provided a forecast for the April to June gu/genna/belg rains, below normal performance of these rains is considered likely. This is expected to exacerbate prevailing food insecurity which resulted from near complete failure of October to December rains in southern pastoral and agro pastoral areas. Due to close to normal sapie (December/January) 2010 rains food security among the dominant root crop, For more information on FEWS NET’s Food Insecurity Severity Scale, please see: www.fews.net/FoodInsecurityScale mainly sweet potatoes growing areas in central and eastern SNNPR is estimated to remain stable Source: FEWS NET and WFP throughout the outlook period. The poor and very poor households normally rely on these harvests, during the March to May lean season. Staple food prices are likely to follow typical seasonal trends throughout the outlook period, though remain higher than the 2005 to 2009 averages given the current harvest and the continued price stabilization measures taken by the government. Seasonal calendar and critical events Source: FEWS NET FEWS NET Washington FEWS NET Ethiopia FEWS NET is a USAID-funded activity. -

By Addis Ababa University Addis Ababa, Ethiopia
ADDIS ABABA UNIVERSITY COLLEGE OF NATURAL AND COMPUTATIONAL SCIENCES SCHOOL OF EARTH SCINCES A FUZZY APPROACH FOR MODELING POTENTIAL WIND FARM AREAS: A CASE OF HITOSA WOREDA, OROMIA REGION, ETHIOPIA By EBISA TESFAYE Advisor: Dr. Binyam Tesfaw A Thesis Submitted to School of Graduate Studies of Addis Ababa University In Partial Fulfillment of the Requirements for the Degree of Masters of Science in Remote sensing and Geo-informatics Addis Ababa University Addis Ababa, Ethiopia June, 2016 ADDIS ABABA UNIVERSITY COLLEGE OF NATURAL AND COMPUTATIONAL SCIENCES SCHOOL OF EARTH SCINCES A FUZZY APPROACH FOR MODELING POTENTIAL WIND FARM AREAS: A CASE OF HITOSA WOREDA, OROMIA REGION, ETHIOPIA By: EBISA TESFAYE DINADE Advisor: Dr. Binyam Tesfaw A Thesis Submitted to School of Graduate Studies of Addis Ababa University In partial fulfillment of the requirements for the degree of Masters of Science in Remote Sensing and Geo-informatics Addis Ababa University Addis Ababa, Ethiopia June, 2016 This is to certify that thesis prepared by EBISA TESFAYE DINADE, entitled: “A Fuzzy Approach for Modeling Potential Wind Farm Areas, A Case of HITOSA Woreda Oromia Region, Ethiopia” and submitted in partial fulfillment of the requirements for the degree of Masters of Science in Remote sensing and Geo-informatics complies with the regulations of the university and meets the accepted standards with respect to the originality and quality. Signed by the Examining committee: Examiner Prof. Balakrishnan M. Signature_________________ Date_______ Dr.Worash Getaneh Signature_________________ Date_______ Advisor Dr. Binyam Tesfaw Signature_________________ Date_______ Chair person Prof. Gezahegn Yirgu Signature_________________ Date_______ ACKNOWLEDGEMENTS The completion of the Master thesis would not have been possible without the assistance of some people, whom I would like to thank.