Current Status of Ectoparasites in Sheep and Management Practices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Protecting Land Tenure Security of Women in Ethiopia: Evidence from the Land Investment for Transformation Program
PROTECTING LAND TENURE SECURITY OF WOMEN IN ETHIOPIA: EVIDENCE FROM THE LAND INVESTMENT FOR TRANSFORMATION PROGRAM Workwoha Mekonen, Ziade Hailu, John Leckie, and Gladys Savolainen Land Investment for Transformation Programme (LIFT) (DAI Global) This research paper was created with funding and technical support of the Research Consortium on Women’s Land Rights, an initiative of Resource Equity. The Research Consortium on Women’s Land Rights is a community of learning and practice that works to increase the quantity and strengthen the quality of research on interventions to advance women’s land and resource rights. Among other things, the Consortium commissions new research that promotes innovations in practice and addresses gaps in evidence on what works to improve women’s land rights. Learn more about the Research Consortium on Women’s Land Rights by visiting https://consortium.resourceequity.org/ This paper assesses the effectiveness of a specific land tenure intervention to improve the lives of women, by asking new questions of available project data sets. ABSTRACT The purpose of this research is to investigate threats to women’s land rights and explore the effectiveness of land certification interventions using evidence from the Land Investment for Transformation (LIFT) program in Ethiopia. More specifically, the study aims to provide evidence on the extent that LIFT contributed to women’s tenure security. The research used a mixed method approach that integrated quantitative and qualitative data. Quantitative information was analyzed from the profiles of more than seven million parcels to understand how the program had incorporated gender interests into the Second Level Land Certification (SLLC) process. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

Religious Sformation Among the Arsi Oromo of Ethiopia Gemechu J. Geda
PILGRIMAGES AND SYNCRETISM: RELIGIOUS TRANSFORMATION AMONG THE ARSI OROMO OF ETHIOPIA GEMECHU J. GEDA A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN RELIGIOUS STUDIES FACULTY O F CULTURAL STUDIES UNIVERSITY OF BAYREUTH GERMANY 23 SEPTEMBER 2013 SUMMARY Currently, the majority of the Arsi Oromo are either Muslims or Christians. However, most of them still practice their traditional beliefs passed down through generations by their forefathers, such as Waaqeffannaa , and attend various rituals related to it . Waaqeffannaa is a religion based on belief in one God known to the Oromo as Waaqa , which according to the Oromo is the creator of the entire universe. The Oromo belief of the existence of Waaqa is based on observing what they call his works, such as the presence of various seasons, rain, sun, darkness, growing of crops, existence of water bodies, mountains, trees and other living things. Contrary to Christianity, Islam, and other religions, Waaqeffannaa does not require the construction of religious houses for the veneration of Waaqa or for thanking him for his good deeds. Instead, the Oromo who are followers of Waaqeffannaa thank Waaqa by travelling to natural physical bodies such as rivers, lakes, forests, and mountains, which they believe are created by Waaqa himself. Waaqeffannaa is believed to be a free will religion, where a believer does not need to calculate in order to obtain certain advantages, such as going to heaven in the afterlife for adhering to Waaqa . To the same effect, a believer would not face some kind of punishment for abandoning Waaqa . -
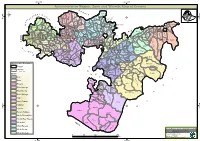
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

OROMIA REGION : Who Does What Where (3W) - WASH Sector (As of 24 June 2013)
(as of 24 June 2013) OROMIA REGION : Who Does What Where (3W) - WASH Sector Tigray Beneshangul Amhara Afar Gumu Afar Amhara Intermon Oxfam: HEKS: CPAR: WVI: Beneshangul Gumu k k k k Dire Dawa Addis Ababa Hareri Intermon Oxfam: Plan Int.: CARE: Gambela Oromia WVE: WVI: Mercy Corps: Somali k ! Wara k k k SNNPR Save the ! CISP: Jarso k ! Childern:k Ababo Horo Abay WVI: Chomen ! CARE: CRS: Guduru ! Dire Dawa ! ! Yaya k k ! Nejo Gulele ! WVI: WVI: Jimma CRS: Meta North ! ! k k Guduru k Goro ! Babo Genete Robi Shewa(R4) Meta ! ! ! Gutu Kersa West ! Gursum ! Jeldu ! ! Wellega TearFund: ! Deder Lalo ! West k Tulo Harari Mieso Asabi East Shewa Sibu Ambo Malka! Wellega Zuria Sire Dendi Addis Ababa Balo ! CRS, WVI ! WVI: k Kelem ! WVI: k Gemechis ! k ! Babile ! Midega CRS: Wellega Save the ! WVI: Habro ! ! West South k Tola ! Wenchi ! Kersana Childern:k ! East Boset Mercy Corps: West Tole Harerge East k Malima Shewa Shewa ! Daro Harerge ZOA: Adama ! Lebu Jeju Boke ! B!ora Guna Gechi HEKS: ! GOAL: Ilubabor ! k k ! Mercy Corps: Dugda! CRS, Hawi WVI: ! k Sekoru Gudina k HEKS k ! ADRA, HEKS ! k Diksis Plan Int.: CISP: Arsi k k Gambela Adami ! Kersa WVI: Tulu Jido ! Dorcas Aid Int.: k ! CRS: IRC: k Kombolcha Degeluna Seru k Jimma ! Tijo Omo Nada GOAL: ! Arsi LVIA: k ! Negele Agencies' locations and ! Save the Shalla Gasera Seweyna area of interventions are Agarfa Children:k GOAL, LIVA, HEKS: ! ! Shashemene Kore ! ! depicted based on the Save the Children: Zuria ! Sinana Siraro Dinsho ! Ginir recent available information. ! ! WVI: Kofele West ! Adaba -

Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group
DRMTechnical Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group Covering 1 Jan to 31 Dec 2016 Prepared by Clusters and NDRMC Introduction The El Niño global climactic event significantly affected the 2015 meher/summer rains on the heels of failed belg/ spring rains in 2015, driving food insecurity, malnutrition and serious water shortages in many parts of the country. The Government and humanitarian partners issued a joint 2016 Humanitarian Requirements Document (HRD) in December 2015 requesting US$1.4 billion to assist 10.2 million people with food, health and nutrition, water, agriculture, shelter and non-food items, protection and emergency education responses. Following the delay and erratic performance of the belg/spring rains in 2016, a Prioritization Statement was issued in May 2016 with updated humanitarian requirements in nutrition (MAM), agriculture, shelter and non-food items and education.The Mid-Year Review of the HRD identified 9.7 million beneficiaries and updated the funding requirements to $1.2 billion. The 2016 HRD is 69 per cent funded, with contributions of $1.08 billion from international donors and the Government of Ethiopia (including carry-over resources from 2015). Under the leadership of the Government of Ethiopia delivery of life-saving and life- sustaining humanitarian assistance continues across the sectors. However, effective humanitarian response was challenged by shortage of resources, limited logistical capacities and associated delays, and weak real-time information management. This Periodic Monitoring Report (PMR) provides a summary of the cluster financial inputs against outputs and achievements against cluster objectives using secured funding since the launch of the 2016 HRD. -

Xerox University Microfilms
INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spiiced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

The Epidemiology of Major Ectoparasites of Sheep and the Effectiveness of the Control Campaign Employed in Tiyo and Diksis Districts, Oromia Region, Ethiopia
Vol. 8(12), pp. 248-254, December 2016 DOI: 10.5897/JVMAH2016.0530 Article Number: AE3605461709 Journal of Veterinary Medicine and ISSN 2141-2529 Copyright © 2016 Animal Health Author(s) retain the copyright of this article http://www.academicjournals.org/JVMAH Full Length Research Paper The epidemiology of major ectoparasites of sheep and the effectiveness of the control campaign employed in Tiyo and Diksis Districts, Oromia Region, Ethiopia Hailegebrael Bedada1*, Gezahegn Alemayehu1, Fikru Gizaw1 and Gemechu Chala2 1College of Veterinary Medicine, Samara University, P. O. Box 132, Semera, Afar, Ethiopia. 2Hawassa University, School of Veterinary Medicine, P. O. Box 05, Hawassa, Ethiopia. Received 24 October, 2016: Accepted 17 November, 2016 A cross-sectional study was conducted from November 2013 to July 2014 with the objectives to determine the prevalence of ectoparasite of sheep in the Tiyo and Diksis districts, determine the effectiveness of control program against sheep ectoparasites in the study area and major risk factors associated with effectiveness of control program. A total of 646 sheep (323 from each districts) were examined for the presence of ectoparasites. From the total sheep examined, 371 (57.43%) were infested with one or more ectoparasites. The ectoparasites identified were lice 49.23%, sheep keds 7.4%, tick 9.75% and mixed infestation 8.98%. Favorable climatic conditions, poor husbandry and animal management, lack of awareness by the farmers, and weak animal health extension services are believed to have contributed for widespread distribution and occurrences of ectoparasites. Even if control campaign is practiced in the study areas, higher prevalence of sheep ectoparasite was recorded. -

ETHIOPIA Food Security Outlook January to June 2011
ETHIOPIA Food Security Outlook January to June 2011 Following the meher harvest, which began in October Figure 1. Current estimated food security outcomes, 2010, food security has generally improved in the January 2011 meher producing parts of the country. However, due to crop damage caused by widespread floods and other weather related shocks the meher harvest is likely to be lower than initially anticipated. The Humanitarian Requirement Documents outlining assistance needs is expected to be released in February 2011. Although the National Meteorology Agency has not provided a forecast for the April to June gu/genna/belg rains, below normal performance of these rains is considered likely. This is expected to exacerbate prevailing food insecurity which resulted from near complete failure of October to December rains in southern pastoral and agro pastoral areas. Due to close to normal sapie (December/January) 2010 rains food security among the dominant root crop, For more information on FEWS NET’s Food Insecurity Severity Scale, please see: www.fews.net/FoodInsecurityScale mainly sweet potatoes growing areas in central and eastern SNNPR is estimated to remain stable Source: FEWS NET and WFP throughout the outlook period. The poor and very poor households normally rely on these harvests, during the March to May lean season. Staple food prices are likely to follow typical seasonal trends throughout the outlook period, though remain higher than the 2005 to 2009 averages given the current harvest and the continued price stabilization measures taken by the government. Seasonal calendar and critical events Source: FEWS NET FEWS NET Washington FEWS NET Ethiopia FEWS NET is a USAID-funded activity. -

Please Find It Enclosed Here with 3 Pages of My CV Date: -06/09/2019
Date: -06/09/2019 Name: Tesfa Demisew Tsegaye Phone: 0923430145 Email: [email protected] TO I would like to apply for the position you want. I was graduated with distinction with a BSc degree in Food Process Engineering from Wolkite University in regular program in 2011 E.C. My academic Program in Food Process Engineering studies emphasize on Supply chain managment, Product development, Control quality and safety, Waste Treatment and management and Food process related projects and also i have complete my apparent time in Asella malt factory and i have 4 year of experience in management at wolkite university peace forum. I have also done my final thesis on Effect of blending ratio on functional and physicochemical property of weaning food from sorghum, kidney beans and maize and complete my work with great achievement (A+). In addition I have experience using several software’s like MICROSOFT (EXCEL, WORD and POWER POINT). I am hardworking, flexible, sociable, capable of supervising a team,presenting and intrinsically motivated. I see my educational background and experience is fitting to the activities your organization is engaged in. I would like to work in your organization and I would be happy to come for test and an interview in any time. Sincerely, Tesfa Demisew Encl: Please find it enclosed here with 3 pages of my CV Date: -06/09/2019 1. PERSONAL INFORMATION Name: Tesfa Demisew Tsegaye Date of birth: September 15, 1995 G.C Birth place: Oromia region, Arsi zone, Asella town Profession: Food Process Engineering Nationality: Ethiopian Sex: Male Marital status: Single Address: Mob: 0923430145 Email: [email protected] 2. -

Clean RCHC Report 2012 Template ENGLISH
RESIDENT / HUMANITARIAN COORDINATOR REPORT ON THE USE OF CERF FUNDS ETHIOPIA UNDERFUNDED EMERGENCY ROUND II 2014 RESIDENT/HUMANITARIAN COORDINATOR Ms. Ahunna Eziakonwa-Onochie REPORTING PROCESS AND CONSULTATION SUMMARY a. Please indicate when the After Action Review (AAR) was conducted and who participated. No formal After Action Review was conducted. However, CERF and CERF projects are regularly discussed at inter-cluster meetings. b. Please confirm that the Resident Coordinator and/or Humanitarian Coordinator (RC/HC) Report was discussed in the Humanitarian and/or UN Country Team and by cluster/sector coordinators as outlined in the guidelines. YES NO UNICEF, FAO, UNFPA, UNHCR, IOM, UNDP, WFP and WHO compiled the draft report and shared with OCHA for review and consolidation. The guidelines and components of reporting were shared with the agencies prior to the preparation of the report. c. Was the final version of the RC/HC Report shared for review with in-country stakeholders as recommended in the guidelines (i.e. the CERF recipient agencies and their implementing partners, cluster/sector coordinators and members and relevant government counterparts)? YES NO The zero draft report was shared with the above agencies for their review and comment; after which the report was amended as per their feedback. The HC also reviewed and endorsed the report. 2 I. HUMANITARIAN CONTEXT TABLE 1: EMERGENCY ALLOCATION OVERVIEW (US$) Total amount required for the humanitarian response: US$403 million Source Amount Breakdown of total CERF 11,593,620 response