Escalation and Morphological Constraints of Antagonistic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2017 AAS Abstracts
2017 AAS Abstracts The American Arachnological Society 41st Annual Meeting July 24-28, 2017 Quéretaro, Juriquilla Fernando Álvarez Padilla Meeting Abstracts ( * denotes participation in student competition) Abstracts of keynote speakers are listed first in order of presentation, followed by other abstracts in alphabetical order by first author. Underlined indicates presenting author, *indicates presentation in student competition. Only students with an * are in the competition. MAPPING THE VARIATION IN SPIDER BODY COLOURATION FROM AN INSECT PERSPECTIVE Ajuria-Ibarra, H. 1 Tapia-McClung, H. 2 & D. Rao 1 1. INBIOTECA, Universidad Veracruzana, Xalapa, Veracruz, México. 2. Laboratorio Nacional de Informática Avanzada, A.C., Xalapa, Veracruz, México. Colour variation is frequently observed in orb web spiders. Such variation can impact fitness by affecting the way spiders are perceived by relevant observers such as prey (i.e. by resembling flower signals as visual lures) and predators (i.e. by disrupting search image formation). Verrucosa arenata is an orb-weaving spider that presents colour variation in a conspicuous triangular pattern on the dorsal part of the abdomen. This pattern has predominantly white or yellow colouration, but also reflects light in the UV part of the spectrum. We quantified colour variation in V. arenata from images obtained using a full spectrum digital camera. We obtained cone catch quanta and calculated chromatic and achromatic contrasts for the visual systems of Drosophila melanogaster and Apis mellifera. Cluster analyses of the colours of the triangular patch resulted in the formation of six and three statistically different groups in the colour space of D. melanogaster and A. mellifera, respectively. Thus, no continuous colour variation was found. -

Antagonistic Coevolution Between the Sexes in a Group of Insects
letters to nature Acknowledgements a central process of evolution, with the potential to shape various We thank the authorities in Madagascar and the Seychelles for permission to conduct interactions between the sexes2,5,6 and their gametes7±8, as well as ®eldwork. We thank J. Brown, T. Peterson, R. Prum, O. Rieppel, L. Trueb and E. Wiley for diversi®cation9, speciation and extinction rates10±12. At the core of comments. C.J.R. and R.A.N. were supported by the National Geographic Society and the this coevolutionary interaction is an arms race between the sexes National Science Foundation, Washington. that can include periods of both escalation and de-escalation of arms13±15. Competing interests statement Theory suggests, however, that the outcome of antagonistic The authors declare that they have no competing ®nancial interests. male±female interactions should remain relatively unchanged Correspondence and requests for materials should be addressed to C.J.R. during an arms race because the build-up of arms in one sex may (e-mail: [email protected]). Sequences are deposited of GenBank under accession numbers be balanced by a build-up in the other (Fig. 1a, 1±2). The AF443224±AF443275. consequences of such arms races on sexual interactions may thus be undetectable, which makes sexually antagonistic coevolution inherently dif®cult to show1±4. Perhaps for this reason, we have no direct empirical evidence for a primary role of arms races in the ................................................................. evolution of sexual interactions in natural systems. Despite an expectation of some evolutionary balance in the level Antagonistic coevolution between of arms between the sexes, one sex may at least temporarily evolve a greater quantity of arms relative to the other (refs 13±15; and Fig. -

A Check List of Gerromorpha (Hemiptera) from India
Rec. zool. Surv. India: 100 (Part 1-2) : 55-97, 2002 A CHECK LIST OF GERROMORPHA (HEMIPTERA) FROM INDIA G. THIRUMALAI Zoological Survey of India, Southern Regional Station, Chennai 600 028. INTRODUCflON The Infraorder Gerromorpha comprises of semi-aquatic bugs characterised by long conspicuous antennae, longer than head and inserted in front of eyes. They are distributed in all kinds of climatic zones, except the coldest and driest parts. This infraorder contains 8 families namely, Gerridae, Veliidae, Hydrometridae, Mesoveliidae, Hebridae, Macroveliidae, Paraphrynoveliidae and Hennatobatidae (Andersen, 1982a). Knowledge of Indian semi-aquatic Hemiptera is limited to the taxonomic preliminaries, recording species from different parts of the country. (Distant, 1903a; 1910 a&b; Annandale, 1919; Bergroth, 1915a; Paiva, 1919a & b; Dover, 1928; Hafiz & Mathai, 1938; Hafiz & Riberio, 1939; Hafiz & Pradhan, 1947; Pradhan, 1950a, b& 1975; Gupta, 1981; Selvanayagam, 1981; Roy et al. 1988; Ghosh et al. 1989; Polhenlus & Starmuhlner, 1990; Bal & Basu, 1994 & 1997; Chen & Zettel, 1999). The revisionary work of Andersen (1975, 1980, 1990 & 1993); Den Boer (1969); Hungerford & Matsuda (1958a & b, 1960, 1962b & 1965); Herring (1961); Polhemus & Andersen (1984); Andersen & Foster (1992); Andersen & Chen (1993); Chen & Nieser (1993a & b); Polhemus & Karunaratne (1993); Polhemus (1994); Polhemus & Polhemus (1994 & 1995a) on a few genera of Gerridae; Lundblad (1936) on the genera Rhagovelia Mayr and Tetraripis Lundblad; Andersen (1981 b, 1983 & 1989); Polhemus -

Sexually Antagonistic Coevolution in a Mating System
Sexually Antagonistic Coevolution in a Mating System: Combining Experimental and Comparative Approaches to Address Evolutionary Processes Author(s): Locke Rowe and Göran Arnqvist Reviewed work(s): Source: Evolution, Vol. 56, No. 4 (Apr., 2002), pp. 754-767 Published by: Society for the Study of Evolution Stable URL: http://www.jstor.org/stable/3061658 . Accessed: 01/10/2012 15:38 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Society for the Study of Evolution is collaborating with JSTOR to digitize, preserve and extend access to Evolution. http://www.jstor.org Evoluition, 56(4), 2002, pp. 754-767 SEXUALLY ANTAGONISTIC COEVOLUTION IN A MATING SYSTEM: COMBINING EXPERIMENTAL AND COMPARATIVE APPROACHES TO ADDRESS EVOLUTIONARY PROCESSES LOCKE ROWE"223 AND GORAN ARNQVIST4 'Department of Zoology, University of Toronto, Toronto, Ontario M5S 3G5, Canada 2Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto, Ontario M5S 2C6 Canada 3E-mail: [email protected] 4Department of Animal Ecology, Evolutionary Biology Centre, University of Uppsala, Norbyvagen 18d, SE-752 36 Uppsala, Sweden Abstract.-We combined experimental and comparative techniques to study the evolution of mating behaviors within in a clade of 15 water striders (Gerris spp.). -

A Comparison of the External Morphology and Functions of Labial Tip Sensilla in Semiaquatic Bugs (Hemiptera: Heteroptera: Gerromorpha)
Eur. J. Entomol. 111(2): 275–297, 2014 doi: 10.14411/eje.2014.033 ISSN 1210-5759 (print), 1802-8829 (online) A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha) 1 2 JOLANTA BROŻeK and HERBERT ZeTTeL 1 Department of Zoology, Faculty of Biology and environmental Protection, University of Silesia, Bankowa 9, PL 40-007 Katowice, Poland; e-mail: [email protected] 2 Natural History Museum, entomological Department, Burgring 7, 1010 Vienna, Austria; e-mail: [email protected] Key words. Heteroptera, Gerromorpha, labial tip sensilla, pattern, morphology, function, apomorphic characters Abstract. The present study provides new data on the morphology and distribution of the labial tip sensilla of 41 species of 20 gerro- morphan (sub)families (Heteroptera: Gerromorpha) obtained using a scanning electron microscope. There are eleven morphologically distinct types of sensilla on the tip of the labium: four types of basiconic uniporous sensilla, two types of plate sensilla, one type of peg uniporous sensilla, peg-in-pit sensilla, dome-shaped sensilla, placoid multiporous sensilla and elongated placoid multiporous sub- apical sensilla. Based on their external structure, it is likely that these sensilla are thermo-hygrosensitive, chemosensitive and mechano- chemosensitive. There are three different designs of sensilla in the Gerromorpha: the basic design occurs in Mesoveliidae and Hebridae; the intermediate one is typical of Hydrometridae and Hermatobatidae, and the most specialized design in Macroveliidae, Veliidae and Gerridae. No new synapomorphies for Gerromorpha were identified in terms of the labial tip sensilla, multi-peg structures and shape of the labial tip, but eleven new diagnostic characters are recorded for clades currently recognized in this infraorder. -

The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V
The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V. Bennett Edwin F. Cook Technical Bulletin 332-1981 Agricultural Experiment Station University of Minnesota St. Paul, Minnesota 55108 CONTENTS PAGE Introduction ...................................3 Key to Adults of Nearctic Families of Semiaquatic Hemiptera ................... 6 Family Saldidae-Shore Bugs ............... 7 Family Mesoveliidae-Water Treaders .......18 Family Hebridae-Velvet Water Bugs .......20 Family Hydrometridae-Marsh Treaders, Water Measurers ...22 Family Veliidae-Small Water striders, Rime bugs ................24 Family Gerridae-Water striders, Pond skaters, Wherry men .....29 Family Ochteridae-Velvety Shore Bugs ....35 Family Gelastocoridae-Toad Bugs ..........36 Literature Cited ..............................37 Figures ......................................44 Maps .........................................55 Index to Scientific Names ....................59 Acknowledgement Sincere appreciation is expressed to the following individuals: R. T. Schuh, for being extremely helpful in reviewing the section on Saldidae, lending specimens, and allowing use of his illustrations of Saldidae; C. L. Smith for reading the section on Veliidae, checking identifications, and advising on problems in the taxon omy ofthe Veliidae; D. M. Calabrese, for reviewing the section on the Gerridae and making helpful sugges tions; J. T. Polhemus, for advising on taxonomic prob lems and checking identifications for several families; C. W. Schaefer, for providing advice and editorial com ment; Y. A. Popov, for sending a copy ofhis book on the Nepomorpha; and M. C. Parsons, for supplying its English translation. The University of Minnesota, including the Agricultural Experi ment Station, is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, creed, color, sex, national origin, or handicap. The information given in this publication is for educational purposes only. -

Biological Diversity, Ecological Health and Condition of Aquatic Assemblages at National Wildlife Refuges in Southern Indiana, USA
Biodiversity Data Journal 3: e4300 doi: 10.3897/BDJ.3.e4300 Taxonomic Paper Biological Diversity, Ecological Health and Condition of Aquatic Assemblages at National Wildlife Refuges in Southern Indiana, USA Thomas P. Simon†, Charles C. Morris‡, Joseph R. Robb§, William McCoy | † Indiana University, Bloomington, IN 46403, United States of America ‡ US National Park Service, Indiana Dunes National Lakeshore, Porter, IN 47468, United States of America § US Fish and Wildlife Service, Big Oaks National Wildlife Refuge, Madison, IN 47250, United States of America | US Fish and Wildlife Service, Patoka River National Wildlife Refuge, Oakland City, IN 47660, United States of America Corresponding author: Thomas P. Simon ([email protected]) Academic editor: Benjamin Price Received: 08 Dec 2014 | Accepted: 09 Jan 2015 | Published: 12 Jan 2015 Citation: Simon T, Morris C, Robb J, McCoy W (2015) Biological Diversity, Ecological Health and Condition of Aquatic Assemblages at National Wildlife Refuges in Southern Indiana, USA. Biodiversity Data Journal 3: e4300. doi: 10.3897/BDJ.3.e4300 Abstract The National Wildlife Refuge system is a vital resource for the protection and conservation of biodiversity and biological integrity in the United States. Surveys were conducted to determine the spatial and temporal patterns of fish, macroinvertebrate, and crayfish populations in two watersheds that encompass three refuges in southern Indiana. The Patoka River National Wildlife Refuge had the highest number of aquatic species with 355 macroinvertebrate taxa, six crayfish species, and 82 fish species, while the Big Oaks National Wildlife Refuge had 163 macroinvertebrate taxa, seven crayfish species, and 37 fish species. The Muscatatuck National Wildlife Refuge had the lowest diversity of macroinvertebrates with 96 taxa and six crayfish species, while possessing the second highest fish species richness with 51 species. -

Systematics, Historical Biogeography and Ecological Phylogenetics in A
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Damgaard Jakob Artikel/Article: Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders 813-822 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders1 J. DAMGAARD Abstract: I hereby review the current knowledge about systematics, historical biogeography and ecolo- gical phylogenetics in the three principal northern temperate genera of water striders Limnoporus STÅL 1868, Aquarius SCHELLENBERG 1800 and Gerris FABRICIUS 1794. Most of the discussion is based on com- parison of a recently published combined analysis tree involving four genetic markers and a morpholo- gical data set with older phylogenetic trees primarily based on manual cladistic optimization of mor- phological characters. Key words: DNA-barcodes, Gerrinae, phylogeography, simultaneous analyses. Introduction nally, water striders show great variation in mating strategies, and morphological and Water striders (Hemiptera-Heteroptera, behavioral adaptations to accomplish or Gerromorpha, Gerridae) are familiar inhab- avoid multiple mating (ANDERSEN 1994, itants of aquatic habitats throughout the 1996; ARNQVIST 1997). The striking diver- Worlds temperate, subtropical, and tropical sity in habitat selection, wing polymorphism regions comprising approximately 640 de- and mating strategies – along with the prac- scribed species in 72 genera (ANDERSEN & tically two dimensional habitat, has made WEIR 2004). Most water striders are found water striders popular objects in studies of in freshwater habitats, such as rivers, behavior, ecology and evolution (SPENCE & streams, lakes and ponds, but a few genera ANDERSEN 1994; ROWE et al. -

Rhagovelia (Hemiptera: Heteroptera: Veliidae)
ACTA BIOLÓGICA COLOMBIANA http://www.revistas.unal.edu.co/index.php/actabiol SEDE BOGOTÁ FACULTAD DE CIENCIAS NOTADEPARTAMENTO BREVE/BRIEF DE BIOLOGÍA NOTE Rhagovelia (Hemiptera: Heteroptera: Veliidae) DE LA CUENCA ALTA DEL RÍO PUTUMAYO (PUTUMAYO, COLOMBIA) Rhagovelia (Hemiptera: Heteroptera: Veliidae) from Upper Basin of the Putumayo River (Putumayo, Colombia) Dora Nancy PADILLA-GIL1. 1 Universidad de Nariño, Departamento de Biología, bloque 3, piso 4, Ciudad Universitaria Torobajo. San Juan de Pasto. Nariño, Colombia. For correspondence. [email protected] Received: 11st January 2016, Returned for revision: 21st February 2016, Accepted: 13th March 2016. Associate Editor: Allan H. Smith Pardo. Citation/Citar este artículo como: Padilla-Gil DN. Rhagovelia (Hemiptera: Heteroptera: Veliidae) de la cuenca alta del Río Putumayo (Putumayo, Colombia). Acta biol. Colomb. 2016;21(3):661-666. DOI: http://dx.doi.org/10.15446/abc.v21n3.55086 RESUMEN Este estudio presenta la composición, diversidad, abundancia, distribución geográfica y altitudinal de las especies del géneroRhagovelia en la cuenca alta del Río Putumayo, Piedemonte sur de la Amazonia, departamento de Putumayo, Colombia. Las estaciones de muestreo fueron establecidas en el rango altitudinal entre los 160 a 590 m. s. n. m., comprendido entre las localidades de Mocoa y Puerto Asis. Las muestras fueron colectadas usando redes entomológicas entre Junio y Noviembre de 2015. En total se encontraron siete especies del género Rhagovelia, pertenecientes a los grupos bisignata y robusta; se describe la forma macróptera de la especie Rhagovelia longipes Gould, 1931. Se amplió el rango geográfico para la mayoría de las especies y se preciso el rango altitudinal de tales especies en el piedemonte de la Amazonía. -
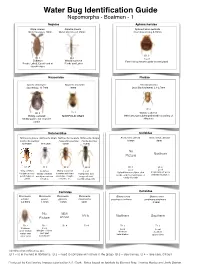
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Marine Insects
UC San Diego Scripps Institution of Oceanography Technical Report Title Marine Insects Permalink https://escholarship.org/uc/item/1pm1485b Author Cheng, Lanna Publication Date 1976 eScholarship.org Powered by the California Digital Library University of California Marine Insects Edited by LannaCheng Scripps Institution of Oceanography, University of California, La Jolla, Calif. 92093, U.S.A. NORTH-HOLLANDPUBLISHINGCOMPANAY, AMSTERDAM- OXFORD AMERICANELSEVIERPUBLISHINGCOMPANY , NEWYORK © North-Holland Publishing Company - 1976 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise,without the prior permission of the copyright owner. North-Holland ISBN: 0 7204 0581 5 American Elsevier ISBN: 0444 11213 8 PUBLISHERS: NORTH-HOLLAND PUBLISHING COMPANY - AMSTERDAM NORTH-HOLLAND PUBLISHING COMPANY LTD. - OXFORD SOLEDISTRIBUTORSFORTHEU.S.A.ANDCANADA: AMERICAN ELSEVIER PUBLISHING COMPANY, INC . 52 VANDERBILT AVENUE, NEW YORK, N.Y. 10017 Library of Congress Cataloging in Publication Data Main entry under title: Marine insects. Includes indexes. 1. Insects, Marine. I. Cheng, Lanna. QL463.M25 595.700902 76-17123 ISBN 0-444-11213-8 Preface In a book of this kind, it would be difficult to achieve a uniform treatment for each of the groups of insects discussed. The contents of each chapter generally reflect the special interests of the contributors. Some have presented a detailed taxonomic review of the families concerned; some have referred the readers to standard taxonomic works, in view of the breadth and complexity of the subject concerned, and have concentrated on ecological or physiological aspects; others have chosen to review insects of a specific set of habitats.