The Genome of the Water Strider Gerris Buenoi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antagonistic Coevolution Between the Sexes in a Group of Insects
letters to nature Acknowledgements a central process of evolution, with the potential to shape various We thank the authorities in Madagascar and the Seychelles for permission to conduct interactions between the sexes2,5,6 and their gametes7±8, as well as ®eldwork. We thank J. Brown, T. Peterson, R. Prum, O. Rieppel, L. Trueb and E. Wiley for diversi®cation9, speciation and extinction rates10±12. At the core of comments. C.J.R. and R.A.N. were supported by the National Geographic Society and the this coevolutionary interaction is an arms race between the sexes National Science Foundation, Washington. that can include periods of both escalation and de-escalation of arms13±15. Competing interests statement Theory suggests, however, that the outcome of antagonistic The authors declare that they have no competing ®nancial interests. male±female interactions should remain relatively unchanged Correspondence and requests for materials should be addressed to C.J.R. during an arms race because the build-up of arms in one sex may (e-mail: [email protected]). Sequences are deposited of GenBank under accession numbers be balanced by a build-up in the other (Fig. 1a, 1±2). The AF443224±AF443275. consequences of such arms races on sexual interactions may thus be undetectable, which makes sexually antagonistic coevolution inherently dif®cult to show1±4. Perhaps for this reason, we have no direct empirical evidence for a primary role of arms races in the ................................................................. evolution of sexual interactions in natural systems. Despite an expectation of some evolutionary balance in the level Antagonistic coevolution between of arms between the sexes, one sex may at least temporarily evolve a greater quantity of arms relative to the other (refs 13±15; and Fig. -

A Check List of Gerromorpha (Hemiptera) from India
Rec. zool. Surv. India: 100 (Part 1-2) : 55-97, 2002 A CHECK LIST OF GERROMORPHA (HEMIPTERA) FROM INDIA G. THIRUMALAI Zoological Survey of India, Southern Regional Station, Chennai 600 028. INTRODUCflON The Infraorder Gerromorpha comprises of semi-aquatic bugs characterised by long conspicuous antennae, longer than head and inserted in front of eyes. They are distributed in all kinds of climatic zones, except the coldest and driest parts. This infraorder contains 8 families namely, Gerridae, Veliidae, Hydrometridae, Mesoveliidae, Hebridae, Macroveliidae, Paraphrynoveliidae and Hennatobatidae (Andersen, 1982a). Knowledge of Indian semi-aquatic Hemiptera is limited to the taxonomic preliminaries, recording species from different parts of the country. (Distant, 1903a; 1910 a&b; Annandale, 1919; Bergroth, 1915a; Paiva, 1919a & b; Dover, 1928; Hafiz & Mathai, 1938; Hafiz & Riberio, 1939; Hafiz & Pradhan, 1947; Pradhan, 1950a, b& 1975; Gupta, 1981; Selvanayagam, 1981; Roy et al. 1988; Ghosh et al. 1989; Polhenlus & Starmuhlner, 1990; Bal & Basu, 1994 & 1997; Chen & Zettel, 1999). The revisionary work of Andersen (1975, 1980, 1990 & 1993); Den Boer (1969); Hungerford & Matsuda (1958a & b, 1960, 1962b & 1965); Herring (1961); Polhemus & Andersen (1984); Andersen & Foster (1992); Andersen & Chen (1993); Chen & Nieser (1993a & b); Polhemus & Karunaratne (1993); Polhemus (1994); Polhemus & Polhemus (1994 & 1995a) on a few genera of Gerridae; Lundblad (1936) on the genera Rhagovelia Mayr and Tetraripis Lundblad; Andersen (1981 b, 1983 & 1989); Polhemus -

Heteroptera: Gerromorpha) in Central Europe
Shortened web version University of South Bohemia in České Budějovice Faculty of Science Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe RNDr. Tomáš Ditrich Ph.D. Thesis Supervisor: Prof. RNDr. Miroslav Papáček, CSc. University of South Bohemia, Faculty of Education České Budějovice 2010 Shortened web version Ditrich, T., 2010: Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe. Ph.D. Thesis, in English. – 85 p., Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic. Annotation Ecology of Veliidae and Mesoveliidae (Hemiptera: Heteroptera: Gerromorpha) was studied in selected European species. The research of these non-gerrid semiaquatic bugs was especially focused on voltinism, overwintering with physiological consequences and wing polymorphism with dispersal pattern. Hypotheses based on data from field surveys were tested by laboratory, mesocosm and field experiments. New data on life history traits and their ecophysiological consequences are discussed in seven original research papers (four papers published in peer-reviewed journals, one paper accepted to publication, one submitted paper and one communication in a conference proceedings), creating core of this thesis. Keywords Insects, semiaquatic bugs, life history, overwintering, voltinism, dispersion, wing polymorphism. Financial support This thesis was mainly supported by grant of The Ministry of Education, Youth and Sports of the Czech Republic No. MSM 6007665801, partially by grant of the Grant Agency of the University of South Bohemia No. GAJU 6/2007/P-PřF, by The Research Council of Norway: The YGGDRASIL mobility program No. 195759/V11 and by Czech Science Foundation grant No. 206/07/0269. Shortened web version Declaration I hereby declare that I worked out this Ph.D. -

Sexually Antagonistic Coevolution in a Mating System
Sexually Antagonistic Coevolution in a Mating System: Combining Experimental and Comparative Approaches to Address Evolutionary Processes Author(s): Locke Rowe and Göran Arnqvist Reviewed work(s): Source: Evolution, Vol. 56, No. 4 (Apr., 2002), pp. 754-767 Published by: Society for the Study of Evolution Stable URL: http://www.jstor.org/stable/3061658 . Accessed: 01/10/2012 15:38 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Society for the Study of Evolution is collaborating with JSTOR to digitize, preserve and extend access to Evolution. http://www.jstor.org Evoluition, 56(4), 2002, pp. 754-767 SEXUALLY ANTAGONISTIC COEVOLUTION IN A MATING SYSTEM: COMBINING EXPERIMENTAL AND COMPARATIVE APPROACHES TO ADDRESS EVOLUTIONARY PROCESSES LOCKE ROWE"223 AND GORAN ARNQVIST4 'Department of Zoology, University of Toronto, Toronto, Ontario M5S 3G5, Canada 2Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto, Ontario M5S 2C6 Canada 3E-mail: [email protected] 4Department of Animal Ecology, Evolutionary Biology Centre, University of Uppsala, Norbyvagen 18d, SE-752 36 Uppsala, Sweden Abstract.-We combined experimental and comparative techniques to study the evolution of mating behaviors within in a clade of 15 water striders (Gerris spp.). -

Observations on the Ecology of Water Striders
* RILEY f : Observations on the Ecology of Water Stridors Zoology M. S. 1912 MS/TV. <>V THE UNIVERSITY OF ILLINOIS LIBRARY ^5 OBSERVATIONS ON THE ECOLOGY OF WATER STRIDERS BY CHARLES FREDERICK CURTIS RILEY A. B. Doane College, 1901 A. B. University of Michigan, 1905 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE IN ZOOLOGY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1912 TH5 \vi_ r-4S UNIVERSITY OF ILLINOIS THE GRADUATE SCHOOL 19JT2J 1 HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY C: 31 C OIL* . ENTITLED IaJoJjjx- ^tX^MlA^ BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF In Charge of Major Work Recommendation concurred in: Committee on Final Examination Digitized by the Internet Archive in 2014 http://archive.org/details/observationsonecOOrile Observations on the Ecology of Water Stridors. Table of Contents. I. Introduction. II. Running Water Habitats 1. Ravines. 2. Springs. 5. Small Temporary Streams. a. Tile-drains and Temporary Streams. 4. Permanent Brooks. a. Behavior During Flood Conditions. b. Drought Conditions. c. Behavior During Conditions of Severe Drought. 5. Creeks. a. Dralnage-di tc h. 6. Rivers. a. Ox-bow Ponds. II. Food and Food Relations. I. Nature and Source of Focd. Food During Captivity. Transportation of Food by Water Currents. Time of Feeding. Response to Water Currents and its Relation to Food. Trial Method of Response to Moving Objects and Its Relation to Food. IV. Summary. V. Bibliography. -3- OESERVATIONS ON THE ECOLOGY OF WATER STRIDERS. I. Introduction. The present paper treats of some of the general ecological relations of water striders. -

Systematics, Historical Biogeography and Ecological Phylogenetics in A
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Damgaard Jakob Artikel/Article: Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders 813-822 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders1 J. DAMGAARD Abstract: I hereby review the current knowledge about systematics, historical biogeography and ecolo- gical phylogenetics in the three principal northern temperate genera of water striders Limnoporus STÅL 1868, Aquarius SCHELLENBERG 1800 and Gerris FABRICIUS 1794. Most of the discussion is based on com- parison of a recently published combined analysis tree involving four genetic markers and a morpholo- gical data set with older phylogenetic trees primarily based on manual cladistic optimization of mor- phological characters. Key words: DNA-barcodes, Gerrinae, phylogeography, simultaneous analyses. Introduction nally, water striders show great variation in mating strategies, and morphological and Water striders (Hemiptera-Heteroptera, behavioral adaptations to accomplish or Gerromorpha, Gerridae) are familiar inhab- avoid multiple mating (ANDERSEN 1994, itants of aquatic habitats throughout the 1996; ARNQVIST 1997). The striking diver- Worlds temperate, subtropical, and tropical sity in habitat selection, wing polymorphism regions comprising approximately 640 de- and mating strategies – along with the prac- scribed species in 72 genera (ANDERSEN & tically two dimensional habitat, has made WEIR 2004). Most water striders are found water striders popular objects in studies of in freshwater habitats, such as rivers, behavior, ecology and evolution (SPENCE & streams, lakes and ponds, but a few genera ANDERSEN 1994; ROWE et al. -

Characterisation of Pristine Polish River Systems and Their Use As Reference Conditions for Dutch River Systems
1830 Rapport 1367.qxp 20-9-2006 16:49 Pagina 1 Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems Rebi Nijboer Piet Verdonschot Andrzej Piechocki Grzegorz Tonczyk´ Malgorzata Klukowska Alterra-rapport 1367, ISSN 1566-7197 Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems Commissioned by the Dutch Ministry of Agriculture, Nature and Food Quality, Research Programme ‘Aquatic Ecology and Fisheries’ (no. 324) and by the project Euro-limpacs which is funded by the European Union under2 Thematic Sub-Priority 1.1.6.3. Alterra-rapport 1367 Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems Rebi Nijboer Piet Verdonschot Andrzej Piechocki Grzegorz Tończyk Małgorzata Klukowska Alterra-rapport 1367 Alterra, Wageningen 2006 ABSTRACT Nijboer, Rebi, Piet Verdonschot, Andrzej Piechocki, Grzegorz Tończyk & Małgorzata Klukowska, 2006. Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems. Wageningen, Alterra, Alterra-rapport 1367. 221 blz.; 13 figs.; 53 tables.; 26 refs. A central feature of the European Water Framework Directive are the reference conditions. The ecological quality status is determined by calculating the distance between the present situation and the reference conditions. To describe reference conditions the natural variation of biota in pristine water bodies should be measured. Because pristine water bodies are not present in the Netherlands anymore, water bodies (springs, streams, rivers and oxbow lakes) in central Poland were investigated. Macrophytes and macroinvertebrates were sampled and environmental variables were measured. The water bodies appeared to have a high biodiversity and a good ecological quality. -
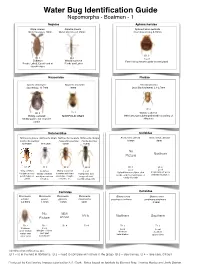
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Diet of Feral Xenopus Laevis (Daudin) in South Wales, U.K
J. Zool., Lond. (1998) 246, 287±298 # 1998 The Zoological Society of London Printed in the United Kingdom Diet of feral Xenopus laevis (Daudin) in South Wales, U.K. G. J. Measey School of Biological Sciences, University of Bristol, Bristol BS8 1UG, U.K. (Accepted 30 March 1998) Abstract African clawed frogs (Xenopus laevis) in a South Wales pond ate a wide variety and size range of prey. Zoobenthos and zooplankton made the greatest contribution to diets, both numerically and by weight. Terrestrial invertebrates made up a small proportion of the diet numerically but a large proportion of the diet mass during the spring and summer. Nektonic prey were present throughout the year but made up a very small proportion of diet. Cannibalism was important when eggs and larvae were present in the pond. Electivity values were consistently positive for chironomids (larvae and pupae) and daphnids but were consistently negative for tubi®cids. In addition, electivity increased for larger sizes and pupae of Chironomus plumosus, but was low for the largest size class (>12 mm). Electivity of other taxa showed an increase when densities of chironomids and daphnids were reduced. Mean sizes of daphnids and cyclopods were consistently larger in frog stomach contents than in the water column, indicating that predation on zooplankton by Xenopus laevis is size-selective. Key words: African clawed frogs, ecology, benthic invertebrates, invasive amphibians, predation, diet selectivity INTRODUCTION analysis (Denton & Beebee, 1994). Most studies show that anurans are typically opportunistic generalist pre- Predation in aquatic communities is now widely consid- dators of invertebrates (Duellman & Trueb, 1986). -

Morphological Study of the Antennal Sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera)
Title: Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera) Author: Agnieszka Nowińska, Jolanta Brożek Citation style: Nowińska Agnieszka, Brożek Jolanta. (2017). Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). "Zoomorphology" (vol. 136, iss. 3 (2017), s. 327-347), doi 10.1007/s00435-017-0354-y Zoomorphology (2017) 136:327–347 DOI 10.1007/s00435-017-0354-y ORIGINAL PAPER Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera) 1 1 A. Nowin´ska • J. Brozek_ Received: 23 January 2017 / Revised: 10 April 2017 / Accepted: 11 April 2017 / Published online: 28 April 2017 Ó The Author(s) 2017. This article is an open access publication Abstract The external morphology and distribution of the The antennae of Gerromorpha belong to the same antennal sensilla of 21 species from five families of morphological type as those found in other heteropteran semiaquatic bugs (Gerromorpha) were examined using insects. The scapus and pecicel are one antennomer while scanning electron microscopy. Nine main types were dis- the flagellum consists of four antennomers (Andersen tinguished based on their morphological structure: sensilla 1982; Schuh and Slater 1995). A significant part of the trichoidea, sensilla chaetica, sensilla leaflike, sensilla sensory system of insects consists of a large number of campaniformia, sensilla coeloconica, sensilla ampullacea, highly diverse organs called sensilla. These sensory organs sensilla basiconica, sensilla placoidea and sensilla bell- are located in the antennae, mouthparts (labium, labial and mouthed. The specific morphological structure of one type maxillary palps and proboscis (a food-sucking tubular of sensilla (bell-mouthed sensilla) was observed only in appendage), genitalia, legs and wings (Peregrine 1972; Aquarius paludum. -
![The A47/A11 Thickthorn Junction Development Consent Order 202[X]](https://docslib.b-cdn.net/cover/5298/the-a47-a11-thickthorn-junction-development-consent-order-202-x-1985298.webp)
The A47/A11 Thickthorn Junction Development Consent Order 202[X]
A47/A11 Thickthorn Junction Environmental Statement Appendix Infrastructure Planning Planning Act 2008 The Infrastructure Planning (Applications: Prescribed Forms and Procedure) Regulations 2009 The A47/A11 Thickthorn Junction Development Consent Order 202[x] ENVIRONMENTAL STATEMENT APPENDICES Appendix 8.3 – Aquatic Macroinvertebrate Survey Report Regulation Number: Regulation 5(2)(a) Planning Inspectorate Scheme TR010037 Reference Application Document Reference TR010037/APP/6.3 BIM Document Reference HE551492-GTY-EBD-000-RP-LB-30010 Author: A47/A11 Thickthorn Junction Project Team, Highways England Version Date Status of Version Rev 0 March 2021 Application Issue Planning Inspectorate Scheme Ref: TR010037 Application Document Ref: TR010037/APP/6.3 A47/A11 Thickthorn Junction Aquatic macroinvertebrate survey report 2020 Carried out for: SWECO Prepared by: Abrehart Ecology The Barn, Bridge Farm Friday Street Brandeston Suffolk IP13 7BP Tel: e-mail: [email protected] www.abrehartecology.com Issue/revision 1 2 Remarks Draft Final Prepared by AJK Date 30/09/2020 01/10/2020 Checked by DB TRA Authorised TRA A47/A11 Thickthorn Junction Aquatic macroinvertebrate survey 2020 Table of Contents 1 Summary ............................................................................................................................................................... 1 2 Introduction ......................................................................................................................................................... 2 3 Methods -

Waterbugs (Heteroptera: Nepomorpha, Gerromorpha) As Sources of Essential N-3
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Siberian Federal University Digital Repository 1 Waterbugs (Heteroptera: Nepomorpha, Gerromorpha) as sources of essential n-3 2 polyunsaturated fatty acids in Central Siberian ecoregions 3 4 Nadezhda N. Sushchik1,2, Yuri A. Yurchenko3,4, Olga E. Belevich3, Galina S. Kalachova1, 5 Angelika A. Kolmakova1, Michail I. Gladyshev1,2 6 7 1 Institute of Biophysics of Siberian Branch of Russian Academy of Sciences, Akademgorodok, 8 50/50, Krasnoyarsk 660036, Russia 9 2 Siberian Federal University, Svobodny av., 79, Krasnoyarsk 660041, Russia 10 3 Institute of Systematics and Ecology of Animals of Siberian Branch of Russian Academy of 11 Sciences, Frunze str. 11, Novosibirsk 630091, Russia 12 4 Institute of Biology, Ecology, Soil, Agriculture and Forest Sciences, Tomsk State University, 13 Lenin av. 36, Tomsk 634050, Russia 14 15 Running title: Fatty-acid composition of Siberian waterbugs 16 Key words: waterbugs, essential fatty acids, Heteroptera, water-land transfers, subsidies; 17 terrestrial consumers 18 19 Correspondence: N.N. Sushchik, Institute of Biophysics, Akademgorodok 50/50, 20 Krasnoyarsk 660036, Russia. Tel.+7 3912 49 52 53 Fax +7 3912 43 34 00 21 E-mail address: [email protected] 22 23 Summary 24 1. Aquatic systems are considered a main source of essential long-chain n-3 polyunsaturated 25 fatty acids (PUFA), which are preferentially synthesized by microalgae and transferred 26 along food chains to terrestrial consumers. Emerging aquatic insects comprise a 27 significant part of this transfer of the essential PUFA from water to land. Quantitative 28 data on PUFA content and composition are available mainly for rheophilic insects while 29 taxa that are characteristic of wetlands and stagnant water bodies, such as aquatic 30 Heteroptera, remain relatively unstudied.