Sexually Antagonistic Coevolution in a Mating System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antagonistic Coevolution Between the Sexes in a Group of Insects
letters to nature Acknowledgements a central process of evolution, with the potential to shape various We thank the authorities in Madagascar and the Seychelles for permission to conduct interactions between the sexes2,5,6 and their gametes7±8, as well as ®eldwork. We thank J. Brown, T. Peterson, R. Prum, O. Rieppel, L. Trueb and E. Wiley for diversi®cation9, speciation and extinction rates10±12. At the core of comments. C.J.R. and R.A.N. were supported by the National Geographic Society and the this coevolutionary interaction is an arms race between the sexes National Science Foundation, Washington. that can include periods of both escalation and de-escalation of arms13±15. Competing interests statement Theory suggests, however, that the outcome of antagonistic The authors declare that they have no competing ®nancial interests. male±female interactions should remain relatively unchanged Correspondence and requests for materials should be addressed to C.J.R. during an arms race because the build-up of arms in one sex may (e-mail: [email protected]). Sequences are deposited of GenBank under accession numbers be balanced by a build-up in the other (Fig. 1a, 1±2). The AF443224±AF443275. consequences of such arms races on sexual interactions may thus be undetectable, which makes sexually antagonistic coevolution inherently dif®cult to show1±4. Perhaps for this reason, we have no direct empirical evidence for a primary role of arms races in the ................................................................. evolution of sexual interactions in natural systems. Despite an expectation of some evolutionary balance in the level Antagonistic coevolution between of arms between the sexes, one sex may at least temporarily evolve a greater quantity of arms relative to the other (refs 13±15; and Fig. -

A Check List of Gerromorpha (Hemiptera) from India
Rec. zool. Surv. India: 100 (Part 1-2) : 55-97, 2002 A CHECK LIST OF GERROMORPHA (HEMIPTERA) FROM INDIA G. THIRUMALAI Zoological Survey of India, Southern Regional Station, Chennai 600 028. INTRODUCflON The Infraorder Gerromorpha comprises of semi-aquatic bugs characterised by long conspicuous antennae, longer than head and inserted in front of eyes. They are distributed in all kinds of climatic zones, except the coldest and driest parts. This infraorder contains 8 families namely, Gerridae, Veliidae, Hydrometridae, Mesoveliidae, Hebridae, Macroveliidae, Paraphrynoveliidae and Hennatobatidae (Andersen, 1982a). Knowledge of Indian semi-aquatic Hemiptera is limited to the taxonomic preliminaries, recording species from different parts of the country. (Distant, 1903a; 1910 a&b; Annandale, 1919; Bergroth, 1915a; Paiva, 1919a & b; Dover, 1928; Hafiz & Mathai, 1938; Hafiz & Riberio, 1939; Hafiz & Pradhan, 1947; Pradhan, 1950a, b& 1975; Gupta, 1981; Selvanayagam, 1981; Roy et al. 1988; Ghosh et al. 1989; Polhenlus & Starmuhlner, 1990; Bal & Basu, 1994 & 1997; Chen & Zettel, 1999). The revisionary work of Andersen (1975, 1980, 1990 & 1993); Den Boer (1969); Hungerford & Matsuda (1958a & b, 1960, 1962b & 1965); Herring (1961); Polhemus & Andersen (1984); Andersen & Foster (1992); Andersen & Chen (1993); Chen & Nieser (1993a & b); Polhemus & Karunaratne (1993); Polhemus (1994); Polhemus & Polhemus (1994 & 1995a) on a few genera of Gerridae; Lundblad (1936) on the genera Rhagovelia Mayr and Tetraripis Lundblad; Andersen (1981 b, 1983 & 1989); Polhemus -

Systematics, Historical Biogeography and Ecological Phylogenetics in A
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Damgaard Jakob Artikel/Article: Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders 813-822 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders1 J. DAMGAARD Abstract: I hereby review the current knowledge about systematics, historical biogeography and ecolo- gical phylogenetics in the three principal northern temperate genera of water striders Limnoporus STÅL 1868, Aquarius SCHELLENBERG 1800 and Gerris FABRICIUS 1794. Most of the discussion is based on com- parison of a recently published combined analysis tree involving four genetic markers and a morpholo- gical data set with older phylogenetic trees primarily based on manual cladistic optimization of mor- phological characters. Key words: DNA-barcodes, Gerrinae, phylogeography, simultaneous analyses. Introduction nally, water striders show great variation in mating strategies, and morphological and Water striders (Hemiptera-Heteroptera, behavioral adaptations to accomplish or Gerromorpha, Gerridae) are familiar inhab- avoid multiple mating (ANDERSEN 1994, itants of aquatic habitats throughout the 1996; ARNQVIST 1997). The striking diver- Worlds temperate, subtropical, and tropical sity in habitat selection, wing polymorphism regions comprising approximately 640 de- and mating strategies – along with the prac- scribed species in 72 genera (ANDERSEN & tically two dimensional habitat, has made WEIR 2004). Most water striders are found water striders popular objects in studies of in freshwater habitats, such as rivers, behavior, ecology and evolution (SPENCE & streams, lakes and ponds, but a few genera ANDERSEN 1994; ROWE et al. -
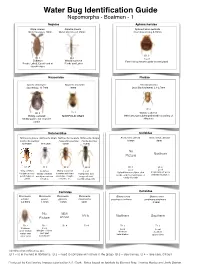
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

The Genome of the Water Strider Gerris Buenoi Reveals Expansions of Gene
bioRxiv preprint doi: https://doi.org/10.1101/242230; this version posted February 11, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The genome of the water strider Gerris buenoi reveals expansions of 2 gene repertoires associated with adaptations to life on the water 3 4 Authors 5 David Armisén1*; Rajendhran Rajakumar2; Markus Friedrich3; Joshua B Benoit4; Hugh M. 6 Robertson5; Kristen A. Panfilio6,7; Seung-Joon Ahn8,9; Monica F. Poelchau10; Hsu Chao11; Huyen 7 Dinh11; HarshaVardhan Doddapaneni11; Shannon Dugan11; Richard A. Gibbs11; Daniel S.T. Hughes11; 8 Yi Han11; Sandra L. Lee11; Shwetha C. Murali12; Donna M. Muzny11; Jiaxin Qu11; Kim C. Worley11; 9 Monica Munoz-Torres13; Ehab Abouheif14; François Bonneton1; Travis Chen14; Li-Mei Chiang10; 10 Christopher P. Childers10; Andrew Graham Cridge15; Antonin Jean Johan Crumière1; Amelie 11 Decaras1; Elise M. Didion4; Elizabeth Duncan15,16; Elena N. Elpidina17; Marie-Julie Favé14; Cédric 12 Finet1, Chris G.C. Jacobs18,19; Alys Jarvela20; Emily J. Jennings4; Jeffery W. Jones3; Maryna P. 13 Lesoway14,21,22; Mackenzie Lovegrove9; Alexander Martynov23; Brenda Oppert24; Angelica Lillico- 14 Ouachour14; Arjuna Rajakumar14; Peter Nagui Refki1,25; Andrew J. Rosendale4; Maria Emilia 15 Santos1; William Toubiana1; Maurijn van der Zee18; Iris M. Vargas Jentzsch6; Aidamalia Vargas 16 Lowman1; Severine Viala1; Stephen Richards11*; and Abderrahman Khila1* 17 18 * Corresponding authors. 19 20 Affiliations 21 1. Institut de Génomique Fonctionnelle de Lyon, Université de Lyon, Université Claude Bernard 22 Lyon 1, CNRS UMR 5242, Ecole Normale Supérieure de Lyon, 46, allée d’Italie, 69364 Lyon Cedex 23 07, France 1 bioRxiv preprint doi: https://doi.org/10.1101/242230; this version posted February 11, 2018. -

Seasonal Life-History Adaptation in the Water
SEASONAL LIFE -HISTORY ADAPTATION IN THE WATER STRIDER GERRIS LACUSTRIS DISSERTATION ZUR ERLANGUNG DES NATURWISSENSCHAFTLICHEN DOKTORGRADES DER BAYERISCHEN JULIUS -MAXIMILIANS -UNIVERSITÄT WÜRZBURG VORGELEGT VON BRENDA PFENNING AUS MARKTHEIDENFELD WÜRZBURG 2008 Eingereicht am: 11. Februar 2008 Mitglieder der Prüfungskommission: Vorsitzender: Prof. Dr. Martin J. Müller Erstgutachter: Prof. Dr. Hans Joachim Poethke Zweitgutachter: Prof. Dr. Jürgen Tautz Tag des Promotionskolloquiums: .............................................. Doktorurkunde ausgehändigt am: ............................................ Table of Contents Chapter 1 General Introduction 1 1.1 LIFE -HISTORY ADAPTATIONS TO VARIATIONS IN SEASON LENGTH ....... 4 1.2 ADAPTATIONS OF WATER STRIDERS TO SEASONALITY .............................. 9 1.3 OUTLINE OF THE THESIS .................................................................................. 12 Chapter 2 Species and Study Area 15 2.1 THE COMMON POND -SKATER GERRIS LACUSTRIS ...................................... 17 2.2 STUDY AREA ....................................................................................................... 20 Chapter 3 Variability in the life history of the water strider Gerris lacustris (Heteroptera: Gerridae) across small spatial scales 23 3.1 INTRODUCTION ................................................................................................. 26 3.2 METHODS ........................................................................................................... 28 Wing length pattern -

Spatial Distribution of Semiaquatic Bugs (Heteroptera: Gerromorpha
Silva Gabreta vol. 14 (3) p. 173–178 Vimperk, 2008 Spatial distribution of semiaquatic bugs (Heteroptera: Gerromorpha) and their wing morphs in a small scale of the Pohořský Potok stream spring area (Novohradské Hory Mts.) Tomáš Ditrich1,2,*, Miroslav Papáček1 & Tomáš Broum3 1Department of Biology, Pedagogical Faculty, University of South Bohemia, Jeronýmova 10, CZ-37115 České Budějovice, Czech Republic 2Department of Ecosystem Biology, Faculty of Science, University of South Bohemia, Branišovská 31, CZ-37005 České Budějovice, Czech Republic 3Větrná 603, CZ-43151 Klášterec nad Ohří, Czech Republic *[email protected] Abstract A survey of semiaquatic bugs (Heteroptera: Gerromorpha) was managed in a small scale area (72 ha; spring area of Pohořský Potok stream, Novohradské Hory Mts., Czech Republic, Central Europe). Species compo- sition of assemblages and rates of their pteromorphs were observed in the relation to selected environmen- tal characteristics – stream velocity, site permanence, water surface coverage and site shading. Gerris gibbifer Schummel, 1832, Gerris lateralis Schummel, 1832 and Velia caprai Tamanini, 1947 are dominant gerromorphan species in the study area. Gerris species are univoltine; bivoltinism of Veliidae species can- not be excluded in this area. A redundancy analysis showed significant effect of stream velocity, site shading and site permanence on species and wing morphs composition of assemblages. Velia caprai mostly occur in shaded habitats with flowing water, whereas other species prefer still water bodies. Gerris lateralis pre- fers shaded sites as the only gerrid. Occurrence of wing morphs of the only notably wing dimorphic speci- es G. lateralis in the study area depends on site permanence. Permanent water bodies are occupied by both macropterous and apterous specimens, temporary sites are almost exclusively colonized by macropterous individuals. -

The Genome of the Water Strider Gerris Buenoi
Armisén et al. BMC Genomics (2018) 19:832 https://doi.org/10.1186/s12864-018-5163-2 RESEARCHARTICLE Open Access The genome of the water strider Gerris buenoi reveals expansions of gene repertoires associated with adaptations to life on the water David Armisén1*, Rajendhran Rajakumar2, Markus Friedrich3, Joshua B. Benoit4, Hugh M. Robertson5, Kristen A. Panfilio6,7, Seung-Joon Ahn8,9, Monica F. Poelchau10, Hsu Chao11, Huyen Dinh11, Harsha Vardhan Doddapaneni11, Shannon Dugan11, Richard A. Gibbs11, Daniel S. T. Hughes11, Yi Han11, Sandra L. Lee11, Shwetha C. Murali12, Donna M. Muzny11, Jiaxin Qu11, Kim C. Worley11, Monica Munoz-Torres13, Ehab Abouheif14, François Bonneton1, Travis Chen14, Li-Mei Chiang10, Christopher P. Childers10, Andrew G. Cridge15, Antonin J. J. Crumière1, Amelie Decaras1, Elise M. Didion4, Elizabeth J. Duncan15,16, Elena N. Elpidina17, Marie-Julie Favé14, Cédric Finet1, Chris G. C. Jacobs18,19, Alys M. Cheatle Jarvela20, Emily C. Jennings4, Jeffery W. Jones3, Maryna P. Lesoway14,21,22, Mackenzie R. Lovegrove15, Alexander Martynov22, Brenda Oppert23, Angelica Lillico-Ouachour14, Arjuna Rajakumar14, Peter Nagui Refki1,24, Andrew J. Rosendale4, Maria Emilia Santos1, William Toubiana1, Maurijn van der Zee18, Iris M. Vargas Jentzsch6, Aidamalia Vargas Lowman1, Severine Viala1, Stephen Richards11* and Abderrahman Khila1* Abstract Background: Having conquered water surfaces worldwide, the semi-aquatic bugs occupy ponds, streams, lakes, mangroves, and even open oceans. The diversity of this group has inspired a range of scientific studies from ecology and evolution to developmental genetics and hydrodynamics of fluid locomotion. However, the lack of a representative water strider genome hinders our ability to more thoroughly investigate the molecular mechanisms underlying the processes of adaptation and diversification within this group. -

The Gerridae Or Water Striders of Oregon and Washington (Hemiptera:Heteroptera)
C5 ,.144 'op. The Gerridae or Water Striders of Oregon and Washington (Hemiptera:Heteroptera) Technical Bulletin 144 AGRICULTURAL EXPERIMENT STATION Oregon State University Corvallis, Oregon April 1982 ---4 Contents Biology and Distribution.....................................................................3 Key to the Adult Gerridae of Oregon and Washington.......................11 Subfamily Gerri nae..................................................................... 16 Genus Gerris Fabricius....................................................... 16 Gerris (Aquarius) remigis Say..................................... 16 Gerris (Gerris) buenoi Kirkaldy................................... 18 Gerris(Gerris)gillettei Lethierryand Severin............ 19 Gerris (Gerris) incognitus Drake and Hottes.............. 20 Gerris(Gerris)incurvatus Drake and Hottes.............. 21 Genus Limnoporus Stal...................................................... 21 Limnoporus notabilis (Drake and Hottes).................. 22 Subfamily Trepobatinae............................................................. 22 Genus Metrobates Uhler..................................................... 22 Metrobates trux infuscatus Usinger.......................... 23 Literature Cited................................................................................... 31 AUTHORS: GaryM. Stonedahl is a doctoral candidate and assistant curator of the Systematic EntomologyLaboratory,Department of Entomology, Oregon State University. JohnD.Lattin is professor of entomology and curatorof -

Water Striders) of Idaho (Heteroptera)
Great Basin Naturalist Volume 49 Number 2 Article 14 4-30-1989 Gerridae (water striders) of Idaho (Heteroptera) R. C. Biggam University of Idaho, Moscow M. A. Brusven University of Idaho, Moscow Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Biggam, R. C. and Brusven, M. A. (1989) "Gerridae (water striders) of Idaho (Heteroptera)," Great Basin Naturalist: Vol. 49 : No. 2 , Article 14. Available at: https://scholarsarchive.byu.edu/gbn/vol49/iss2/14 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. GERRIDAE (WATER STRIDERS) OF IDAHO (HETEROPTERA) 1 R. C. Biggam" and M. A. Brusven" Abstract. —A biosystematic study on the Gerridae of Idaho was undertaken to clarify and describe the taxonomy, species distribution, and biology of this aquatic hemipteran family. Three genera and 7 species were collected in the state. Keys to three genera and 10 species are provided. General descriptions, diagnoses, and distributional ranges are given for species occurring within and adjacent to Idaho. Special interest in aquatic and subaquatic (Scudder 1969, 1971), brackish coastal waters Heteroptera by the authors and recently pub- (Vepsalainen 1973, Andersen 1975, Cobben lished papers on the Gerridae of Montana 1960), and the open ocean (Andersen and Pol- (Roemhild 1976) and Oregon and Washington hemus 1976). Five species of the genus Halo- (Stonedahl and Lattin 1982) prompted this bates have been collected hundreds of miles taxonomic study on the Gerridae of Idaho. -
Gerris Lacustris (Linaeus 1758) and Gerris Costae (Herrich-Schäffer 1850) Species - Habitat Relations on Mountainous Tributaries of Vişeu River (Maramureş, Romania)
Transylv. Rev. Syst. Ecol. Res. 15.1 (2013), "The Wetlands Diversity" DOI: 10.2478/trser-2013-0002 11 GERRIS LACUSTRIS (LINAEUS 1758) AND GERRIS COSTAE (HERRICH-SCHÄFFER 1850) SPECIES - HABITAT RELATIONS ON MOUNTAINOUS TRIBUTARIES OF VIŞEU RIVER (MARAMUREŞ, ROMANIA) Horea OLOSUTEAN * and Daniela Minodora ILIE ** * “Lucian Blaga” University of Sibiu, Faculty of Sciences, Dr. Ion Raţiu Street 5-7, Sibiu, Sibiu County, Romania, RO-550012, [email protected] ** “Lucian Blaga” University of Sibiu, Faculty of Sciences, Dr. Ion Raţiu Street 5-7, Sibiu, Sibiu County, Romania, RO-550012, [email protected] KEYWORDS: Gerris lacustris, Gerris costae, Vişeu River basin, habitat characteristics. ABSTRACT Semi aquatic Heteroptera species from some mountainous tributaries of the Vişeu River were collected and their relations with habitat variables were investigated. Only two species, Gerris lacustris and Gerris costae were found, either one or both species, in almost half of the investigated sampling stations. Correlation analysis between samplings and habitat conditions showed that Gerris lacustris prefers small deep ponds or river banks with steep slopes and is easily adaptable to habitat changes, while Gerris costae is mostly found in large marshes with low, stagnant water and high amounts of vegetation. Both species are relatively tolerant to human impact in their habitat, Gerris lacustris more so. The two species are negatively correlated to each other, as an expression of competition between them. Principal Component Analysis resulted in two dominant factors explaining almost 60% of the habitat variation, and their graphic representation proved the observed correlations. ZUSAMMENFASSUNG: Die Art-Habitat-Beziehungen von Gerris lacustris Linaeus 1758 und Gerris costae Herrich-Schäffer 1853 an den montanen Zuflüssen des Vişeu (Maramuresch, Rumänien). -

Escalation and Morphological Constraints of Antagonistic
Escalation and Morphological Constraints of Antagonistic Armaments in Water Striders Antonin Jean Johan Crumière, David Armisen, Aïdamalia Vargas-Lowman, Martha Kubarakos, Felipe Ferraz Figueiredo Moreira, Abderrahman Khila To cite this version: Antonin Jean Johan Crumière, David Armisen, Aïdamalia Vargas-Lowman, Martha Kubarakos, Felipe Ferraz Figueiredo Moreira, et al.. Escalation and Morphological Constraints of Antagonistic Arma- ments in Water Striders. Frontiers in Ecology and Evolution, Frontiers Media S.A, 2019, 7, pp.12. 10.3389/fevo.2019.00215. hal-02392029 HAL Id: hal-02392029 https://hal.archives-ouvertes.fr/hal-02392029 Submitted on 25 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License ORIGINAL RESEARCH published: 18 June 2019 doi: 10.3389/fevo.2019.00215 Escalation and Morphological Constraints of Antagonistic Armaments in Water Striders Antonin Jean Johan Crumière 1†, David Armisén 1†, Aïdamalia Vargas-Lowman 1, Martha Kubarakos 1†, Felipe Ferraz Figueiredo Moreira 2 and Abderrahman Khila 1* Edited by: 1 Institut de Génomique Fonctionnelle de Lyon, CNRS UMR 5242, Ecole Normale Supérieure de Lyon, Université de Lyon, Jingchun Li, Université Claude Bernard Lyon 1, Lyon, France, 2 Laboratório de Biodiversidade Entomológica, Instituto Oswaldo Cruz, University of Colorado Boulder, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil United States Reviewed by: Sexual conflict may result in the escalating coevolution of sexually antagonistic traits.