Plankton Diversity Zooplankton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Temperature in Survival of the Polyp Stage of the Tropical Rhizostome Jelly®Sh Cassiopea Xamachana
Journal of Experimental Marine Biology and Ecology, L 222 (1998) 79±91 The role of temperature in survival of the polyp stage of the tropical rhizostome jelly®sh Cassiopea xamachana William K. Fitt* , Kristin Costley Institute of Ecology, Bioscience 711, University of Georgia, Athens, GA 30602, USA Received 27 September 1996; received in revised form 21 April 1997; accepted 27 May 1997 Abstract The life cycle of the tropical jelly®sh Cassiopea xamachana involves alternation between a polyp ( 5 scyphistoma) and a medusa, the latter usually resting bell-down on a sand or mud substratum. The scyphistoma and newly strobilated medusa (5 ephyra) are found only during the summer and early fall in South Florida and not during the winter, while the medusae are found year around. New medusae originate as ephyrae, strobilated by the polyp, in late summer and fall. Laboratory experiments showed that nematocyst function, and the ability of larvae to settle and metamorphose change little during exposure to temperatures between 158C and up to 338C. However, tentacle length decreased and ability to transfer captured food to the mouth was disrupted at temperatures # 188C. Unlike temperate-zone species of scyphozoans, which usually over-winter in the polyp or podocyst form when medusae disappear, this tropical species has cold-sensitive scyphistomae and more temperature-tolerant medusae. 1998 Elsevier Science B.V. Keywords: Scyphozoa; Jelly®sh; Cassiopea; Temperature; Life history 1. Introduction The rhizostome medusae of Cassiopea xamachana are found throughout the Carib- bean Sea, with their northern limit of distribution on the southern tip of Florida. Unlike most scyphozoans these jelly®sh are seldom seen swimming, and instead lie pulsating bell-down on sandy or muddy substrata in mangroves or soft bottom bay habitats, giving rise to the common names ``mangrove jelly®sh'' or ``upside-down jelly®sh''. -
Appendicularia of CTAW
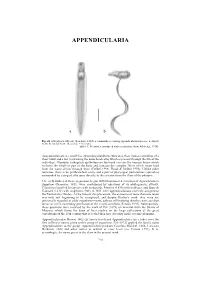
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Population and Spatial Dynamics Mangrove Jellyfish Cassiopeia Sp at Kenya’S Gazi Bay
American Journal of Life Sciences 2014; 2(6): 395-399 Published online December 31, 2014 (http://www.sciencepublishinggroup.com/j/ajls) doi: 10.11648/j.ajls.20140206.20 ISSN: 2328-5702 (Print); ISSN: 2328-5737 (Online) Population and spatial dynamics mangrove jellyfish Cassiopeia sp at Kenya’s Gazi bay Tsingalia H. M. Department of Biological Sciences, Moi University, Box 3900-30100, Eldoret, Kenya Email address: [email protected] To cite this article: Tsingalia H. M.. Population and Spatial Dynamics Mangrove Jellyfish Cassiopeia sp at Kenya’s Gazi Bay. American Journal of Life Sciences. Vol. 2, No. 6, 2014, pp. 395-399. doi: 10.11648/j.ajls.20140206.20 Abstract: Cassiopeia, the upside-down or mangrove jellyfish is a bottom-dwelling, shallow water marine sycophozoan of the phylum Cnidaria. It is commonly referred to as jellyfish because of its jelly like appearance. The medusa is the dominant phase in its life history. They have a radial symmetry and occur in shallow, tropical lagoons, mangrove swamps and sandy mud falls in tropical and temperate regions. In coastal Kenya, they are found only in one specific location in the Gazi Bay of the south coast. There are no documented studies on this species in Kenya. The objective of this study was to quantify the spatial and size-class distribution, and recruitment of Cassiopeia at the Gazi Bay. Ten 50mx50m quadrats were randomly placed in an estimated study area of 6.4ha to cover about 40 percent of the total study area. A total of 1043 individual upside-down jellyfish were sampled. In each quadrat, all jellyfish encountered were sampled individually. -

The Evolution of Siphonophore Tentilla for Specialized Prey Capture in the Open Ocean
The evolution of siphonophore tentilla for specialized prey capture in the open ocean Alejandro Damian-Serranoa,1, Steven H. D. Haddockb,c, and Casey W. Dunna aDepartment of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520; bResearch Division, Monterey Bay Aquarium Research Institute, Moss Landing, CA 95039; and cEcology and Evolutionary Biology, University of California, Santa Cruz, CA 95064 Edited by Jeremy B. C. Jackson, American Museum of Natural History, New York, NY, and approved December 11, 2020 (received for review April 7, 2020) Predator specialization has often been considered an evolutionary makes them an ideal system to study the relationships between “dead end” due to the constraints associated with the evolution of functional traits and prey specialization. Like a head of coral, a si- morphological and functional optimizations throughout the organ- phonophore is a colony bearing many feeding polyps (Fig. 1). Each ism. However, in some predators, these changes are localized in sep- feeding polyp has a single tentacle, which branches into a series of arate structures dedicated to prey capture. One of the most extreme tentilla. Like other cnidarians, siphonophores capture prey with cases of this modularity can be observed in siphonophores, a clade of nematocysts, harpoon-like stinging capsules borne within special- pelagic colonial cnidarians that use tentilla (tentacle side branches ized cells known as cnidocytes. Unlike the prey-capture apparatus of armed with nematocysts) exclusively for prey capture. Here we study most other cnidarians, siphonophore tentacles carry their cnidocytes how siphonophore specialists and generalists evolve, and what mor- in extremely complex and organized batteries (3), which are located phological changes are associated with these transitions. -

US Fish & Wildlife Service Seabird Conservation Plan—Pacific Region
U.S. Fish & Wildlife Service Seabird Conservation Plan Conservation Seabird Pacific Region U.S. Fish & Wildlife Service Seabird Conservation Plan—Pacific Region 120 0’0"E 140 0’0"E 160 0’0"E 180 0’0" 160 0’0"W 140 0’0"W 120 0’0"W 100 0’0"W RUSSIA CANADA 0’0"N 0’0"N 50 50 WA CHINA US Fish and Wildlife Service Pacific Region OR ID AN NV JAP CA H A 0’0"N I W 0’0"N 30 S A 30 N L I ort I Main Hawaiian Islands Commonwealth of the hwe A stern A (see inset below) Northern Mariana Islands Haw N aiian Isla D N nds S P a c i f i c Wake Atoll S ND ANA O c e a n LA RI IS Johnston Atoll MA Guam L I 0’0"N 0’0"N N 10 10 Kingman Reef E Palmyra Atoll I S 160 0’0"W 158 0’0"W 156 0’0"W L Howland Island Equator A M a i n H a w a i i a n I s l a n d s Baker Island Jarvis N P H O E N I X D IN D Island Kauai S 0’0"N ONE 0’0"N I S L A N D S 22 SI 22 A PAPUA NEW Niihau Oahu GUINEA Molokai Maui 0’0"S Lanai 0’0"S 10 AMERICAN P a c i f i c 10 Kahoolawe SAMOA O c e a n Hawaii 0’0"N 0’0"N 20 FIJI 20 AUSTRALIA 0 200 Miles 0 2,000 ES - OTS/FR Miles September 2003 160 0’0"W 158 0’0"W 156 0’0"W (800) 244-WILD http://www.fws.gov Information U.S. -

The Puzzling Occurrence of the Upside-Down Jellyfish Cassiopea
ZOOLOGIA 37: e50834 ISSN 1984-4689 (online) zoologia.pensoft.net RESEARCH ARTICLE The puzzling occurrence of the upside-down jellyfishCassiopea (Cnidaria: Scyphozoa) along the Brazilian coast: a result of several invasion events? Sergio N. Stampar1 , Edgar Gamero-Mora2 , Maximiliano M. Maronna2 , Juliano M. Fritscher3 , Bruno S. P. Oliveira4 , Cláudio L. S. Sampaio5 , André C. Morandini2,6 1Departamento de Ciências Biológicas, Laboratório de Evolução e Diversidade Aquática, Universidade Estadual Paulista “Julio de Mesquita Filho”. Avenida Dom Antônio 2100, 19806-900 Assis, SP, Brazil. 2Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo. Rua do Matão, Travessa 14 101, 05508-090 São Paulo, SP, Brazil. 3Instituto do Meio Ambiente de Alagoas. Avenida Major Cícero de Góes Monteiro 2197, 57017-515 Maceió, AL, Brazil. 4Instituto Biota de Conservação, Rua Padre Odilon Lôbo, 115, 57038-770 Maceió, Alagoas, Brazil 5Laboratório de Ictiologia e Conservação, Unidade Educacional de Penedo, Universidade Federal de Alagoas. Avenida Beira Rio, 57200-000 Penedo, AL, Brazil. 6Centro de Biologia Marinha, Universidade de São Paulo. Rodovia Manoel Hypólito do Rego, km 131.5, 11612-109 São Sebastião, SP, Brazil. Corresponding author: Sergio N. Stampar ([email protected]) http://zoobank.org/B879CA8D-F6EA-4312-B050-9A19115DB099 ABSTRACT. The massive occurrence of jellyfish in several areas of the world is reported annually, but most of the data come from the northern hemisphere and often refer to a restricted group of species that are not in the genus Cassiopea. This study records a massive, clonal and non-native population of Cassiopea and discusses the possible scenarios that resulted in the invasion of the Brazilian coast by these organisms. -

Eurochordata and Cephalochordata of Persian Gulf & Oman See
Subphylum Urochordata : Tunicates Class Ascidacea Class Larvacea Class Thaliacea Subphylum Cephalochordata : Amphioxus Phylum Chordata : 1 - Subphylum Urochordata 2 Subphylum Cephalochordata (Subphylum Urochordata : Tunicates ) Class Ascidiacea Class Larvacea Class Thaliacea (Subphylum Cephalochordata ) Class Ascidiacea Ascidia Kowalevsky Patricia Kott D. lane David George & Gordon Paterson Herdmania momus savigny Herdmania momus kiamanensis Didemnum candidum savigny Phallusia nigra savigny Styela canopus savigny Class Ascidiacea : Order : Aplousobranchia Family : Polyclinidae : Aplidium cf. rubripunctum C.Monniot & F. Monniot 1997 Polyclinum constellatum Savigny, 1816 Family Didemnidae Didemnum candidum Savigny, 1816 Didemnum cf. granulatum Tokioka, 1954 Didemnum obscurum F. Monniot 1969 Didemnum perlucidum F. Monniot, 1983 Didemnum sp. Didemnum yolky C.Monniot & F. Monniot 1997 Diplosoma listerianum Milne Edwards, 1841 Order : Phlebobranchia Family : Ascidiidae Ascidia sp. Phallusia julinea Sluiter, 1915 Phallusia nigra Savigny, 1816 Order : Stolidobranchia Family : Styelidae Botryllus gregalis Sluiter, 1898 Botryllus niger Herdman, 1886 Botryllus sp. Eusynstyela cf. hartmeyeri Michaelsen, 1904 Polyandrocarpa sp. Styela canopus Savigny, 1816 Symplegma bahraini C.Monniot & F. Monniot 1997 Symplegma brakenhielmi Michaelsen, 1904 Family pyuridae Herdmania momus Savigny, 1816 Pyura curvigona Tokioka, 1950 Pyurid sp. Class Larvacea Larva Seymour Sewell Phylum Chordata Subphylum Urochordata * Class Larvacea Order Copelata Family Fritillariidae -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -
![Cassiopea Andromeda (Forsskål, 1775) [Cnidaria: Scyphozoa: Rhizostomea]](https://docslib.b-cdn.net/cover/5281/cassiopea-andromeda-forssk%C3%A5l-1775-cnidaria-scyphozoa-rhizostomea-815281.webp)
Cassiopea Andromeda (Forsskål, 1775) [Cnidaria: Scyphozoa: Rhizostomea]
Aquatic Invasions (2006) Volume 1, Issue 3: 196-197 DOI 10.3391/ai.2006.1.3.18 © 2006 The Author(s) Journal compilation © 2006 REABIC (http://www.reabic.net) This is an Open Access article Short communication A new record of an alien jellyfish from the Levantine coast of Turkey - Cassiopea andromeda (Forsskål, 1775) [Cnidaria: Scyphozoa: Rhizostomea] Cem Çevik*, Itri Levent Erkol and Benin Toklu Çukurova University, Faculty of Fisheries,Department of Marine Biology, Adana, 01330, Turkey Email: [email protected] *Corresponding author Received 17 July 2006; accepted in revised form 5 September 2006 Abstract To date, three alien scyphozoan species were reported from the Eastern Mediterranean, but only one, Rhopilema nomadica, was reported from the Turkish coast. Recently, a second alien scyphozoan, Cassiopea andromeda, was collected on 20 July 2005 in Iskenderun Bay, SE Turkey. Key words: Cassiopea andromeda, Scyphozoa, Levant Sea, Turkey, Iskenderun Bay, alien species Cassiopea andromeda (Forsskål, 1775) is the Factory (36°11.200'N and 36°43.100'E) on first Erythrean scyphomedusan species reported 20.07.2005. The site was sampled monthly since from the Mediterranean following the opening of 2002. the Suez Canal. It had been collected in the Suez Two of the six individuals found which have a Canal in 1886 (Galil et al. 1990), and soon after mean umbrella length of 5 cm were taken for from Cyprus (Maas 1903). Nearly half a century further investigation in the laboratory, and were later it was found in Neokameni, a small determined as C. andromeda (Figure 1) volcanic island near Thira, in the southern according to the identification key given by Galil Aegean Sea (Schäffer 1955), and later in et al. -

Tropical Marine Invertebrates CAS BI 569 Phylum Chordata by J
Tropical Marine Invertebrates CAS BI 569 Phylum Chordata by J. R. Finnerty Porifera Ctenophora Cnidaria Deuterostomia Ecdysozoa Lophotrochozoa Chordata Arthropoda Annelida Hemichordata Onychophora Mollusca Echinodermata *Nematoda *Platyhelminthes Acoelomorpha Calcispongia Silicispongiae PROTOSTOMIA Phylum Chordata subPhylum VERTEBRATA subPhylum UROCHORDATA class Ascidaceae—the “sea squirts” class Larvaceae—the larvaceans class Thaliaceae—the salps subPhylum CEPHALOCHORDATA—the “lancelets” Subphylum Subphylum Subphylum Phylum Phylum Vertebrata Urochordata Cephalochordata ECHINODERMATA HEMICHORDATA Phylum CHORDATA Notochord Blastopore -> anus Dorsal hollow nerve cord Radial / equal cleavage Pharyngeal gill slits Coelom forms by enterocoely Endostyle Cross-section through a Chick embryo During Neurulation Subphylum Vertebrata Ordovician Origins—Jawless fishes vertebral column paired appendages post-anal tail Jawed fishes, gnathostomes late Ordovician, early Silurian jaws early Devonian / late Devonian tetrapods / amniotes Permian (299-251) Carboniferous subphylum VERTEBRATA (359-299) gnathostomes (with jaws) 1. Vertebral column Devonian 2. Paired appendages (416-359) 3. Jaws 4. Post anal tail Silurian (444-416) Bony fishes Cartilaginous fishes Paleozoic (543-225) Fishes with jaws & paired fins Ray-finned & Lobe-finned fishes Ordivician Jawless fishes (488-444) Vertebrate origins Jawless fishes phylum CHORDATA Cambrian 1. Dorsal nerve cord (542-488) Non-vert chordates “Cambrian explosion” 2. Notochord Rapid appearance of most 3. Gill slits Animal phyla in fossil record Including Phylum Chordata 4. Endostyle Permian (299-251) 4 3 2 1 1.Pre-mammal-like reptiles 2. Snake-lizard ancestor 3.Crocodile-Bird-Dino ancestor 4. Turtles Carboniferous (359-299) amphibians Early reptiles. Devonian (416-359) Vertebrates invade land “Age of amphibians” Silurian (444-416) Bony fishes AMNIOTES Cartilaginous fishes Paleozoic (543-225) Fishes with jaws & paired fins 1. -

Biochemical Characterization of Cassiopea Andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea
marine drugs Article Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea Gianluca De Rinaldis 1,2,† , Antonella Leone 1,3,*,† , Stefania De Domenico 1,4 , Mar Bosch-Belmar 5 , Rasa Slizyte 6, Giacomo Milisenda 7 , Annalisa Santucci 2, Clara Albano 1 and Stefano Piraino 3,4 1 Institute of Sciences of Food Production (CNR-ISPA, Unit of Lecce), National Research Council, Via Monteroni, 73100 Lecce, Italy; [email protected] (G.D.R.); [email protected] (S.D.D.); [email protected] (C.A.) 2 Department of Biotechnology Chemistry and Pharmacy (DBCF), Università Degli Studi Di Siena, Via A. Moro, 53100 Siena, Italy; [email protected] 3 Consorzio Nazionale Interuniversitario per le Scienze del Mare (CoNISMa, Local Unit of Lecce), Via Monteroni, 73100 Lecce, Italy; [email protected] 4 Department of Biological and Environmental Sciences and Technologies (DiSTeBA), Campus Ecotekne, University of Salento, Via Lecce-Monteroni, 73100 Lecce, Italy 5 Laboratory of Ecology, Department of Earth and Marine Sciences (DiSTeM), University of Palermo, 90128 Palermo, Italy; [email protected] 6 Department of Fisheries and New Biomarine Industry, SINTEF Ocean, Brattørkaia 17C, 7010 Trondheim, Norway; [email protected] 7 Centro Interdipartimentale della Sicilia, Stazione Zoologica Anton Dohrn, Lungomare Cristoforo Colombo, 90142 Palermo, Italy; [email protected] Citation: De Rinaldis, G.; Leone, A.; * Correspondence: [email protected]; Tel.: +39-0832-422615 De Domenico, S.; Bosch-Belmar, M.; † These authors contributed equally to this work. Slizyte, R.; Milisenda, G.; Santucci, A.; Albano, C.; Piraino, S. -

Bibliography on the Scyphozoa with Selected References on Hydrozoa and Anthozoa
W&M ScholarWorks Reports 1971 Bibliography on the Scyphozoa with selected references on Hydrozoa and Anthozoa Dale R. Calder Virginia Institute of Marine Science Harold N. Cones Virginia Institute of Marine Science Edwin B. Joseph Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Marine Biology Commons, and the Zoology Commons Recommended Citation Calder, D. R., Cones, H. N., & Joseph, E. B. (1971) Bibliography on the Scyphozoa with selected references on Hydrozoa and Anthozoa. Special scientific eporr t (Virginia Institute of Marine Science) ; no. 59.. Virginia Institute of Marine Science, William & Mary. https://doi.org/10.21220/V59B3R This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. BIBLIOGRAPHY on the SCYPHOZOA WITH SELECTED REFERENCES ON HYDROZOA and ANTHOZOA Dale R. Calder, Harold N. Cones, Edwin B. Joseph SPECIAL SCIENTIFIC REPORT NO. 59 VIRGINIA INSTITUTE. OF MARINE SCIENCE GLOUCESTER POINT, VIRGINIA 23012 AUGUST, 1971 BIBLIOGRAPHY ON THE SCYPHOZOA, WITH SELECTED REFERENCES ON HYDROZOA AND ANTHOZOA Dale R. Calder, Harold N. Cones, ar,d Edwin B. Joseph SPECIAL SCIENTIFIC REPORT NO. 59 VIRGINIA INSTITUTE OF MARINE SCIENCE Gloucester Point, Virginia 23062 w. J. Hargis, Jr. April 1971 Director i INTRODUCTION Our goal in assembling this bibliography has been to bring together literature references on all aspects of scyphozoan research. Compilation was begun in 1967 as a card file of references to publications on the Scyphozoa; selected references to hydrozoan and anthozoan studies that were considered relevant to the study of scyphozoans were included.