Macro- and Microstructure of the Superior Cervical Ganglion in Dogs, Cats and Horses During Maturation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Sympathetic and the Parasympathetic Nervous System
The sympathetic and the parasympathetic nervous system Zsuzsanna Tóth, PhD Institute of Anatomy, Histology and Embryology Semmelweis University The role of the autonomic nervous system Claude Bernard • „milieu intérieur” concept; every organism lives in its internal environment that is constant and independent form the external environment Walter Bradford Cannon homeostasis; • an extension of the “milieu interieur” concept • consistence in an open system requires mechanisms that act to maintain that consistency • steady-state conditions require that any tendency toward change automatically meets with factors that resist that change • regulating systems that determine the homeostatic state : o autonomic nervous system ( sympathetic, parasympathetic, enteral) o endocrine system General structure of the autonomic nervous system craniosacral thoracolumbar Anatomy Neurotransmittersof the gut autonomic nervous system. symp. gangl pregangl. fiber pregangl. postgangl. fiber fiber (PoR) PoR enteral ganglion PoR PoR smooth muscle smooth muscle Kuratani S Development 2009;136:1585-1589 Sympathetic activation: Fight or flight reaction • energy mobilization • preparation for escape, or fight vasoconstriction • generalized Parasympathetic activation: adrenal • energy saving and restoring • „rest and digest” system • more localized vasoconstriction Paravertebral ganglia and the sympathetic chains pars cervicalis superius ganglion medium cervicale stellatum pars vertebrae • from the base of the skull to the caudal end thoracalis thoracalis of the sacrum • paravertebral ganglia (ganglia trunci sympathici) • rami interganglionares pars vertebrae • the two chains fuses at the ganglion impar abdominalis lumbalis sacrum pars pelvina foramen sacralia anteriora ganglion impar Anatomy of the cervical part of the sympathetic trunk superior cervical ganglion • behind the seath of the carotid, fusiform ggl. cervicale superius • IML T1-3 vegetative motoneurons- preganglionic fibers truncus symp. -

THE MAIN PERIPHERAL CONNECTIONS of the HUMAN SYMPATHETIC NERVOUS SYSTEM by T
THE MAIN PERIPHERAL CONNECTIONS OF THE HUMAN SYMPATHETIC NERVOUS SYSTEM By T. K. POTTS, M.B., CH.M. (SYDNEY)1 BIIE recent investigation (5,7) of the functional significance of the sympathetic system by 1)r N. D). itoyle and Professor J. I. Hunter has revealed the necessity for a re-examination of the anatomy of the human sympathetic system. Ini particular the operations of ramisectioni (7, 8) devised by Dr Royle, in collabora- tion with Professor Hunter, call for a more exact determination of the precise position and topographical relations of the sympathetic cord and its ram? cotitnunicantes than at present is available. The dissection described ill this note was undertaken primarily to provide the surgeon with this guidance. In this matter, two regions stand out as having assumed an added interest ill the light of recent research. I refer to those regions associated with the operations known as cervical, and lumbar sympathetic ramisection, which are performed to remove the rigidity of the musculature of the extremities ill spastic paralysis (2,3,4,5, 7, 8, 9,10). As a description of the rari commnunicantes necessarily involves some mention of the arrangement of corresponding ganglia, this will be done in considering the various regions. To facilitate demonstration, the services of Miss D. Harrison were procured and, under my guidance, faithful repro- dluetions of the dissection were made by her. The dissection has been mounted, and placed in the Wilson. Museum of Anatomy, at the Medical School, Uni- versity of Sydney. The cervical portion of the sympathetic is characterized by the absence of segmental ganglia, and of white rami comnimunicantes. -

CVM 6100 Veterinary Gross Anatomy
2010 CVM 6100 Veterinary Gross Anatomy General Anatomy & Carnivore Anatomy Lecture Notes by Thomas F. Fletcher, DVM, PhD and Christina E. Clarkson, DVM, PhD 1 CONTENTS Connective Tissue Structures ........................................3 Osteology .........................................................................5 Arthrology .......................................................................7 Myology .........................................................................10 Biomechanics and Locomotion....................................12 Serous Membranes and Cavities .................................15 Formation of Serous Cavities ......................................17 Nervous System.............................................................19 Autonomic Nervous System .........................................23 Abdominal Viscera .......................................................27 Pelvis, Perineum and Micturition ...............................32 Female Genitalia ...........................................................35 Male Genitalia...............................................................37 Head Features (Lectures 1 and 2) ...............................40 Cranial Nerves ..............................................................44 Connective Tissue Structures Histologic types of connective tissue (c.t.): 1] Loose areolar c.t. — low fiber density, contains spaces that can be filled with fat or fluid (edema) [found: throughout body, under skin as superficial fascia and in many places as deep fascia] -

The Axatomy of the Autonomic Nervous System in the Dog1
THE AXATOMY OF THE AUTONOMIC NERVOUS SYSTEM IN THE DOG1 NICHOLAS JAMES AIIZERES Ucpartnitiit of Aiintoiiry, Cnzvemtty of Xtclizgaii Scliool of ;2/cdicmc. Ann Arbor, Mtclatgan ELEVEN FIGURES INTRODUCTTOX I<iiowledgc clerivccl from cmprimeiits on tlie clog has not infrequently been applied to man without considering dif- fcmnccs in aiiatoniy. This is c~spcciallytrue of the autonomic nervous systeni, oiily parts of which have b:mi described in the adult clog. The cardiac ncli'vcs \\'ere described by Sc1iuran.- Iew ( '27) ant1 Soniclez ( '39), the gcw~alplan of the abrloniirial ancl sacral regions by Trumble ( '34), tlic lunibosacral trunk by Alehler, Fisclier and Alexander ( '52), the vagi lsy Hilsa- beck aid Hill ( '50) and BIcC'rca and D'hrcy ( '%), ant1 the urogenital plexuses by Schal~adasch( '26) aiicl Ncdowar ( '2.3. The present study was undertaken to fill gaps in previous clescriptions ancl to present an over-all view of the autonomic iierrous system in the (log, esclutliiig the cephalic region. In the pi-c~seiitiiivcstigation the XI< terminology has been used iiistpad of the usual RNA familiar in human anatomy loccause tlic study was based on the clog. Sincc a dog is a pronograde niamnial the terms cranial aiid caudal seeni nior(t appropriat r tliaii the ESA terms superior and inferior. Tiiis paper is n condensation of a clissei t:ition snbniittctl in paytial fulfillmc.~it of tlrc rcqiiireiiieuts for the drgrce of Doctor of Pliilosopliy in the University of Rlicliigaii. I wish to tlimik Dr. It. T. Wootlhuine for Ills interest and aclriec. For the supply of nlatciial tlie author vihlies to e\-pr('\s his appreciatioir to mcmbc~is of the 1)ep:irtiiiciits of P1iTsiolog.y :iiiil PIiar~u:~cologrof tlic Viiirersity of hliclrigan. -

THE ANATOMY of the SYMPATHETHIC TRUNKS in MAN by MARTIN WRETE Histological Department, the University of Uppsala, Sweden
[ 448 ] THE ANATOMY OF THE SYMPATHETHIC TRUNKS IN MAN BY MARTIN WRETE Histological Department, The University of Uppsala, Sweden INTRODUCTION Even a cursory study of the anatomical descriptions of the cervical parts of the sympathetic trunks given in modern text-books or articles discloses that, now as earlier, great confusion exists with respect to terminology. This applies even to monographs and more specialized presentations. The primary cause of this confusion is the very marked variability of the trunks in the neck region, which gives wide scope for arbitrary interpretations of the arrangement; some uncertainty about the terminology and notation of other parts of the trunks also persists. It is true that the terms to be used for the sympathetic nervous system were fixed by the International Anatomical Nomenclature Committee (Nomina Anatomica, Paris, 1955). This does not, however, prevent some of the individual terms being used to denote different anatomical units, and for practical reasons (such as limiting printing costs) comprehensive explanations could not always be given in the annota- tions to the Parisian Nomina Anatomica. As one of the three members of the Sub- Committee responsible for the nomenclature of the peripheral nervous system, I wish to define more exactly my views on the terminology adopted for the sympathetic trunks. I also take this opportunity of revising a few terms I used in certain papers published some twenty years ago. In Nomina Anatomica the term truncus sympathicus is followed by the names of its ganglia, ganglia trunci sympathici, as well as of its connecting rami interganglio- nares. But, also under the heading ganglia trunci sympathici, the term ganglia intermedia is used to denote ganglia on the rami communicantes and certain ganglia on the trunks in the rami interganglionares between the other ganglia-namely the ganglion cervicale superius, ganglion cervicale medium, ganglion cervicothoracicum (s. -

Neurobiology of Visceral Pain
Neurobiology of Visceral Pain Definition Pain arising from the internal organs of the body: • Heart, great vessels, and perivascular structures (e.g., lymph nodes) • Airway structures (pharynx, trachea, bronchi, lungs, pleura) • Gastrointestinal tract (esophagus, stomach, small intestine, colon, rectum) • Upper-abdominal structures (liver, gallbladder, biliary tree, pancreas, spleen) • Urological structures (kidneys, ureters, urinary bladder, urethra) • Reproductive organs (uterus, ovaries, vagina, testes, vas deferens, prostate) • Omentum, visceral peritoneum Clinical Features of Visceral Pain Key features associated with pain from the viscera include diffuse localization, an unreliable association with pathology, and referred sensations. Strong autonomic and emotional responses may be evoked with minimal sensation. Referred pain has two components: (1) a localization of the site of pain generation to somatic tissues with nociceptive processing at the same spinal segments (e.g., chest and arm pain from cardiac ischemia) and (2) a sensitization of these segmental tissues (e.g., kidney stones may cause the muscles of the lateral torso to become tender to palpation). These features are in contrast to cutaneous pain, which is well localized and features a graded stimulus-response relationship. Anatomy of Neurological Structures Pathways for visceral sensation are diffusely organized both peripherally and centrally. Primary afferent nerve fibers innervating viscera project into the central nervous system via three pathways: (1) in the vagus nerve and its branches; (2) within and alongside sympathetic efferent fiber pathways (sympathetic chain and splanchnic branches, including greater, lesser, least, thoracic, and lumbar branches); and (3) in the pelvic nerve (with parasympathetic efferents) and its branches. Passage through the peripheral ganglia occurs with potential synaptic contact (e.g., celiac, superior mesenteric, and hypogastric nerves). -

Jemds.Com Original Research Article
Jemds.com Original Research Article MIDDLE CERVICAL GANGLION AND VERTEBRAL GANGLION- CONTROVERSIES UNVEILED Vandana Latha Raveendran1, K. Girijakumari Kamalamma2 1Assistant Professor, Department of Anatomy, Government Medical College, Thiruvananthapuram, Kerala, India. 2Professor and HOD, Department of Anatomy, Sree Mookambika Institute of Medical Sciences, Kulasekharam, Tamilnadu, India. ABSTRACT BACKGROUND Great divergences are there regarding the occurrence and nomenclature of Middle Cervical Ganglion (MCG) and Vertebral Ganglion (VG) in human cervical sympathetic chain. The lack of a detailed knowledge of its branching pattern and vascular relations will lead to iatrogenic injury complicating many surgical procedures in the neck. MATERIALS AND METHODS The study was done on 50 cervical sympathetic chains by bilateral neck dissection of 25 adult cadavers in the Department of Anatomy, Medical College, Thiruvananthapuram. The vascular relations, branches and dimensions of Superior Cervical Ganglion (SCG), Inferior Cervical Ganglion (ICG), Stellate Ganglion and other intermediate ganglia were carefully dissected out and studied. RESULTS SCG was seen in 100% cases, Inferior Cervical Ganglion (ICG) in 72% cases and stellate ganglion in 28% chains. Out of the two intermediate ganglia between SCG and ICG, one seen in close relation to Inferior Thyroid Artery (ITA) was concluded as MCG (44%) and another ganglion which was always over vertebral artery was concluded to be VG (72%). The branches arising from the ganglia were Gray Rami Communicantes (GRC), vascular branches and medial visceral branches. Vertebral nerve from VG was present in 5.6% cases. CONCLUSION The VG is more frequently present than MCG. Also, comparative studies between the dimensions of MCG and VG when seen alone or together show no significant relation suggesting that both are independent ganglia and one is not a detached part of another. -

Hadeel Abdullah Nadeen AL-Falooji Dena Kofahi Mohammad Hesham
Hadeel Abdullah Nadeen AL-Falooji Dena Kofahi Mohammad Hesham 0 | P a g e Nerves ON THE POSTERIOR ABDOMINAL WALL The Lumbar Plexus The lumbar plexus, which is one of the main nervous pathways supplying the lower limb, is formed in the psoas major muscle -in the abdomen- from the anterior rami of the upper four lumbar spinal nerves (L1-L4). Its branches emerge from the lateral₁ and medial₂ borders of the muscle and from its anterior₃ surface. Branches of the Lumbar Plexus - The iLiohypogastric nerve₁, iLioinguinal nerve₂, Lateral cutaneous nerve of the thigh₃, and femoraL nerve₄ emerge from the Lateral border of the psoas, in that order from above downward. - The obturator nerve₁ and lumbosacral trunk₂ emerge from the medial border of the psoas major. - The genitofemoral₁ nerve emerges from the anterior surface of the psoas major. The lumbosacral trunk: It is formed from the L4 (from the lumbar plexus) and L5 (from the sacral plexus) nerve roots (i.e. the fourth lumbar nerve gives off branches to the sacral plexus forming the lumbosacral trunk). - The ventral (anterior) rami of L1 form the iliohypogastric₁ and ilioinguinal₂ nerves which run between the transversus abdominis muscle and abdominal internal oblique muscle. Then: 1. The iliohypogastric nerve (L1) gives off several motor branches to abdominal muscles and a sensory branch to the skin of the lower part of the anterior abdominal wall above the pubic symphysis. 2. The ilioinguinal nerve (L1) pierces the posterior wall of the inguinal canal and runs along with the spermatic cord (through the canal) to supply the skin of the groin and the scrotum or labium majus. -
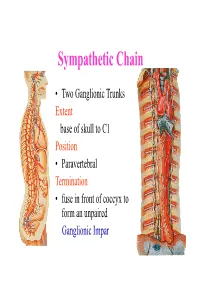
Sympathetic Chain – Cervical Part
Sympathetic Chain • Two Ganglionic Trunks Extent base of skull to C1 Position • Paravertebral Termination • fuse in front of coccyx to form an unpaired Ganglionic Impar Sympathetic Chain •3 Ganglia in cervical part •11Ganglia in thoracic Part •4 lumbar Ganglia •4 Sacral ganglia Sympathetic Chain-cervical part • lie Behind Carotid Sheath and • in front of Longus colli & Longus Capitis muscles • Initially no. of Sympathetic ganglia correspond to no. of Spinal Nerves • Later • Superior formed by fusion of upper 4 cervical Ganglia • Middle by 5th and 6th •Inferiorby joining of 7th and 8th cervical ganglia Sympathetic Chain – Cervical Part • Cervical Part Ganglia Superior Cervical ganglia Middle Cervical Ganglia Inferior Cervical ganglia Sometimes Inferior cervical and first Thoracic fuse to form a Cervico-Thoracic or Stellate Ganglia Sympathetic Chain – Cervical Part • Do not receive white rami communicantes from cervical spinal segments • LAT. HORN CELLS OF T1-T5 PROVIDE PRE- GANGLIONIC FIBRES • Gives grey rami communicantes to all 8 cervical nerves Sympathetic Chain – Cervical Part • GANGLION • Contains-multipolar post ganglionic neurons & few interneurons (chromaffin or SIF cells*) • *modulate activities of post ganglionic neurons by dopamine • SYMPATHETIC TRUNK conveys pre & post ganglionic motor & sensory fibres between ganglia SUPERIOR CERVICAL GANGLION • Largest ,fusiform, 2.5cm length • Fuses upper four cervical ganglia • Situation- opposite C2 &C3 Vertebrae behind ICA& infont of l. capitis • Receives pre ganglionic fibres mostly from upper three thracic segments • BRANCHES (all convey post ganglionic fibres & some sensory fibres) SUPERIOR CERVICAL GANGLION • BRANCHES • Lateral-grey rami comm. to C1- C4 nerves &(C5-C8) • Medial-laryngo-pharyngeal - cardiac(no pain fibr.) Anterior-ramify around CCA,ECA & its branches Ascending-INTERNAL CAROTID NERVE -carotido-tympanic -deep petrosal -communicating(v,iii,iv,v,&vi) -nervus conarii (pineal gland) Term.communicating(ant. -

Vertebral Arteries Bilaterally Passing Through Stellate (Cervicothoracic) Ganglion B
Folia Morphol. Vol. 79, No. 3, pp. 621–626 DOI: 10.5603/FM.a2019.0115 C A S E R E P O R T Copyright © 2020 Via Medica ISSN 0015–5659 journals.viamedica.pl Vertebral arteries bilaterally passing through stellate (cervicothoracic) ganglion B. Chaudhary1, P.R. Tripathy2, M.R. Gaikwad2 1All India Institute of Medical Science, Patna, India 2All India Institute of Medical Science, Bhubaneswar, India [Received: 27 August 2019; Accepted: 5 October 2019] Vertebral artery is a branch of the first part of subclavian artery. Vertebral artery arising from the aortic arch most commonly presents on the left side. The cervical part of sympathetic trunk is closely related to the vertebral artery in the cervical region. Though lots of variations regarding anomalous origin, course of vertebral artery is reported in the literature, here we present a rare anomaly in which ver- tebral artery after originating from aortic arch is passing through stellate ganglia and it enters into the transverse foramina of higher cervical vertebra (C5). Such variation should be kept in mind by anaesthetist during stellate ganglion block in order to relieve intractable pain in central nervous system lesion. Surgeons should keep this anomaly in mind during cervical spine surgery otherwise vertebral artery may get injured leading to haemorrhage. (Folia Morphol 2020; 79, 3: 621–626) Key words: aortic arch, haemodynamic, Horner syndrome, sympathetic ganglia, vertebral artery INTRODUCTION hemisphere [15]. It is divided into four segments. The In normal anatomy of aortic arch and its great first segment (V1) extends from its origin up to its vessels, vertebral artery (VA) arises from the first part entry into C6 transverse foramen, the second part (V2) of subclavian artery (SA). -

Neuroanatomy ©
NEUROANATOMY 2016 NEUROANATOMY © LC Hudson, DVM, PhD Professor Emerita of Anatomy North Carolina State University College of Veterinary Medicine Raleigh NC 27607 [email protected] I. CENTRAL NERVOUS SYSTEM All 5 divisions of the brain are involved with the function of the eyes and/or adnexa - in either conscious/response pathways or reflex pathways. The divisions are the telencephalon (cerebral hemispheres), diencephalon (thalamus, hypothalamus, metathalamus (lateral geniculate nuclei)), mesencephalon (midbrain (pretectal, oculomotor, trochlear nuclei, parasym. nucleus of CN III)), metencephalon (cerebellum and pons (vestibular nuclei, spinal tract of CN V)) and myelencephalon (medulla oblongata (abducens, facial, vestibular nuclei)). The more cranial spinal cord is also involved with function of the eyelids through sensory innervation into the cervical spinal cord, and through sympathetic autonomic function (T1-T3 segments). The spinal cord caudal to T3 is not involved with eye functions. II. PERIPHERAL NERVOUS SYSTEM The majority of the 12 pr. of cranial nerves have some function with the eyes/adnexa- CNs II, III, IV, V (ophthalmic and maxillary branches), VI, VII, and VIII including appropriate sensory and/or autonomic ganglia of these cranial nerves. Retrograde tracing studies in cats showed that some cervical spinal nerves have sensory projections from the eyelids even though apparently physically distant. Autonomic sympathetic fibers are projected via Tl-T3 spinal nerves into the sympathetic trunk, traveling through the neck in the vagosympathetic trunk and synapsing in the cranial cervical ganglion. Postganglionic fibers then travel along blood vessels and with other cranial nerves to reach the globe. III. NOMENCLATURE Veterinary ophthalmologists tend to use human/zoological nomenclature including eponyms such as Meibomian gland, Descemet's membrane, and canal of Schlemm; probably because of the intense use of human literature. -

A Study on Cervical Symphatetic Chain and Raynauds Phenomenon
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 7, Issue 6 (May.- Jun. 2013), PP 52-55 www.iosrjournals.org A Study on Cervical Symphatetic Chain and Raynauds Phenomenon. Balaji Karunagaran1,Thotakura Balaji 2 ,Venkateshgobi Veerappan3 Aruna subramaniyam4, Murugesan Karthikeyan5 1Department of Anatomy, R.V.S Dental college, Coimbatore, Tamilnadu. 2Department of Anatomy, Chettinad Hospital & Research Institute, Kanchipuram. 3Department of Anatomy, Sivaraj Institute of medical sciences, Salem Tamilnadu. 4Department of Anatomy,IndiraGandhai institute of medical sciences, Pondychery.. 5Microbiology Unit, Faculty of Medicine, Quest International University Perak ,Ipoh,Malasiya. Abstract: The cervical part of the sympathetic trunk contains three interconnected ganglia - superior, middle and cervicothoracic. They send grey rami communicantes to all the cervical spinal nerves. The study was conducted on 157 cadavers (99 Male & 58 Female) bilaterally. Superior cervical ganglion was observed in all the cases. Middle cervical ganglion was found in 58.5% of the cases which lies between the common carotid artery in front and loop of inferior thyroid artery behind. Ansasubclavia extends from middle cervical ganglion to inferior cervical ganglion in 12.5% of the cases. Inferior cervical ganglion was observed in the 45% of the cases. The stellate ganglion was observed in 55% of the cases. The variations of the cervical sympathetic trunk were noted and photographed. Stimulated by the need of surgery, anatomy of the cervical sympathetic chain has acquired increasing importance. To diminish the potential risk of injury during surgery better surgical methods are to be developed. I. Introduction The base of neck is a junction for thorax, neck and the upper limb.