PRA Report Acidovorax
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Whole Issue
ORGANISATION EUROPEENNE EUROPEAN AND MEDITERRANEAN ET MEDITERRANEENNE PLANT PROTECTION POUR LA PROTECTION DES PLANTES ORGANIZATION EPPO Reporting Service NO. 7 PARIS, 2009-07-01 CONTENTS _____________________________________________________________________ Pests & Diseases 2009/128 - First record of Monilinia fructicola in Switzerland 2009/129 - First report of Gymnosporangium yamadae in the USA 2009/130 - Isolated finding of Diaporthe vaccinii in the Netherlands 2009/131 - Hymenoscyphus albidus is the teleomorph of Chalara fraxinea 2009/132 - A new real-time PCR assay to detect Chalara fraxinea 2009/133 - Acidovorax citrulli: addition to the EPPO Alert List 2009/134 - First report of Chrysanthemum stunt viroid in Finland 2009/135 - First report of Tomato chlorotic dwarf viroid in Finland 2009/136 - Transmission of Tomato chlorotic dwarf viroid by tomato seeds 2009/137 - Potato spindle tuber viroid detected on tomatoes growing near infected Solanum jasminoides in Liguria, Italy 2009/138 - Strawberry vein banding virus detected in Italy 2009/139 - Incursion of Tomato spotted wilt virus in Finland 2009/140 - Incursion of Bemisia tabaci in Finland 2009/141 - Incursion of Liriomyza huidobrensis in Finland 2009/142 - New data on quarantine pests and pests of the EPPO Alert List 2009/143 - Quarantine List of Moldova 2009/144 - EPPO report on notifications of non-compliance CONTENTS _______________________________________________________________________Invasive Plants 2009/145 - New records of Hydrocotyle ranunculoides in France 2009/146 - Report of the Bern Convention meeting on Invasive Alien Species, Brijuni National Park (HR), 2009-05-05/07 2009/147 - “Plant invasion in Italy, an overview”: a new publication 2009/148 - New data on alien plants in Italy 2009/149 - Lists of invasive alien plants in Russia 2009/150 - The new NOBANIS Newsletter 2009/151 - The Convention on Biological Diversity magazine “business 2010” dedicated to invasive alien species 1, rue Le Nôtre Tel. -

Short Communication Biofilm Formation and Degradation of Commercially Available Biodegradable Plastic Films by Bacterial Consortiums in Freshwater Environments
Microbes Environ. Vol. 33, No. 3, 332-335, 2018 https://www.jstage.jst.go.jp/browse/jsme2 doi:10.1264/jsme2.ME18033 Short Communication Biofilm Formation and Degradation of Commercially Available Biodegradable Plastic Films by Bacterial Consortiums in Freshwater Environments TOMOHIRO MOROHOSHI1*, TAISHIRO OI1, HARUNA AISO2, TOMOHIRO SUZUKI2, TETSUO OKURA3, and SHUNSUKE SATO4 1Department of Material and Environmental Chemistry, Graduate School of Engineering, Utsunomiya University, 7–1–2 Yoto, Utsunomiya, Tochigi 321–8585, Japan; 2Center for Bioscience Research and Education, Utsunomiya University, 350 Mine-machi, Utsunomiya, Tochigi 321–8505, Japan; 3Process Development Research Laboratories, Plastics Molding and Processing Technology Development Group, Kaneka Corporation, 5–1–1, Torikai-Nishi, Settsu, Osaka 556–0072, Japan; and 4Health Care Solutions Research Institute Biotechnology Development Laboratories, Kaneka Corporation, 1–8 Miyamae-cho, Takasago-cho, Takasago, Hyogo 676–8688, Japan (Received March 5, 2018—Accepted May 28, 2018—Published online August 28, 2018) We investigated biofilm formation on biodegradable plastics in freshwater samples. Poly(3-hydroxybutyrate-co-3- hydroxyhexanoate) (PHBH) was covered by a biofilm after an incubation in freshwater samples. A next generation sequencing analysis of the bacterial communities of biofilms that formed on PHBH films revealed the dominance of the order Burkholderiales. Furthermore, Acidovorax and Undibacterium were the predominant genera in most biofilms. Twenty-five out of 28 PHBH-degrading -

Proteomic and Phenotypic Analyses of a Putative Glycerol-3-Phosphate Dehydrogenase Required for Virulence in Acidovorax Citrulli
Plant Pathol. J. 37(1) : 36-46 (2021) https://doi.org/10.5423/PPJ.OA.12.2020.0221 The Plant Pathology Journal pISSN 1598-2254 eISSN 2093-9280 ©The Korean Society of Plant Pathology Research Article Open Access Fast Track Proteomic and Phenotypic Analyses of a Putative Glycerol-3-Phosphate Dehydrogenase Required for Virulence in Acidovorax citrulli Minyoung Kim1, Jongchan Lee1, Lynn Heo1, Sang Jun Lee 2*, and Sang-Wook Han 1* 1Department of Plant Science and Technology, Chung-Ang University, Anseong 17546, Korea 2Department of Systems Biotechnology, Chung-Ang University, Anseong 17546, Korea (Received on December 9, 2020; Revised on December 28, 2020; Accepted on December 29, 2020) Acidovorax citrulli (Ac) is the causal agent of bacterial presence of glycerol, indicating that GlpdAc is involved fruit blotch (BFB) in watermelon, a disease that poses in glycolysis. AcΔglpdAc(EV) also displayed higher cell- a serious threat to watermelon production. Because of to-cell aggregation than the wild-type bacteria, and tol- the lack of resistant cultivars against BFB, virulence erance to osmotic stress and ciprofloxacin was reduced factors or mechanisms need to be elucidated to control and enhanced in the mutant, respectively. These results the disease. Glycerol-3-phosphate dehydrogenase is the indicate that GlpdAc is involved in glycerol metabolism enzyme involved in glycerol production from glucose and other mechanisms, including virulence, demon- during glycolysis. In this study, we report the functions strating that the protein has pleiotropic effects. Our of a putative glycerol-3-phosphate dehydrogenase in study expands the understanding of the functions of Ac (GlpdAc) using comparative proteomic analysis and proteins associated with virulence in Ac. -

Acidovorax Citrulli
Bulletin OEPP/EPPO Bulletin (2016) 46 (3), 444–462 ISSN 0250-8052. DOI: 10.1111/epp.12330 European and Mediterranean Plant Protection Organization Organisation Europe´enne et Me´diterrane´enne pour la Protection des Plantes PM 7/127 (1) Diagnostics Diagnostic PM 7/127 (1) Acidovorax citrulli Specific scope Specific approval and amendment This Standard describes a diagnostic protocol for Approved in 2016-09. Acidovorax citrulli.1 This Standard should be used in conjunction with PM 7/76 Use of EPPO diagnostic protocols. strain, were mainly isolated from non-watermelon, cucurbit 1. Introduction hosts such as cantaloupe melon (Cucumis melo var. Acidovorax citrulli is the causal agent of bacterial fruit cantalupensis), cucumber (Cucumis sativus), honeydew blotch and seedling blight of cucurbits (Webb & Goth, melon (Cucumis melo var. indorus), squash and pumpkin 1965; Schaad et al., 1978). This disease was sporadic until (Cucurbita pepo, Cucurbita maxima and Cucurbita the late 1980s (Wall & Santos, 1988), but recurrent epi- moschata) whereas Group II isolates were mainly recovered demics have been reported in the last 20 years (Zhang & from watermelon (Walcott et al., 2000, 2004; Burdman Rhodes, 1990; Somodi et al., 1991; Latin & Hopkins, et al., 2005). While Group I isolates were moderately 1995; Demir, 1996; Assis et al., 1999; Langston et al., aggressive on a range of cucurbit hosts, Group II isolates 1999; O’Brien & Martin, 1999; Burdman et al., 2005; Har- were highly aggressive on watermelon but moderately ighi, 2007; Holeva et al., 2010; Popovic & Ivanovic, 2015). aggressive on non-watermelon hosts (Walcott et al., 2000, The disease is particularly severe on watermelon (Citrullus 2004). -

Plant-Derived Benzoxazinoids Act As Antibiotics and Shape Bacterial Communities
Supplemental Material for: Plant-derived benzoxazinoids act as antibiotics and shape bacterial communities Niklas Schandry, Katharina Jandrasits, Ruben Garrido-Oter, Claude Becker Contents Supplemental Tables 2 Supplemental Table 1. Phylogenetic signal lambda . .2 Supplemental Table 2. Syncom strains . .3 Supplemental Table 3. PERMANOVA . .6 Supplemental Table 4. PERMANOVA comparing only two treatments . .7 Supplemental Table 5. ANOVA: Observed taxa . .8 Supplemental Table 6. Observed diversity means and pairwise comparisons . .9 Supplemental Table 7. ANOVA: Shannon Diversity . 11 Supplemental Table 8. Shannon diversity means and pairwise comparisons . 12 Supplemental Table 9. Correlation between change in relative abundance and change in growth . 14 Figures 15 Supplemental Figure 1 . 15 Supplemental Figure 2 . 16 Supplemental Figure 3 . 17 Supplemental Figure 4 . 18 1 Supplemental Tables Supplemental Table 1. Phylogenetic signal lambda Class Order Family lambda p.value All - All All All All 0.763 0.0004 * * Gram Negative - Proteobacteria All All All 0.817 0.0017 * * Alpha All All 0 0.9998 Alpha Rhizobiales All 0 1.0000 Alpha Rhizobiales Phyllobacteriacae 0 1.0000 Alpha Rhizobiales Rhizobiacaea 0.275 0.8837 Beta All All 1.034 0.0036 * * Beta Burkholderiales All 0.147 0.6171 Beta Burkholderiales Comamonadaceae 0 1.0000 Gamma All All 1 0.0000 * * Gamma Xanthomonadales All 1 0.0001 * * Gram Positive - Actinobacteria Actinomycetia Actinomycetales All 0 1.0000 Actinomycetia Actinomycetales Intrasporangiaceae 0.98 0.2730 Actinomycetia Actinomycetales Microbacteriaceae 1.054 0.3751 Actinomycetia Actinomycetales Nocardioidaceae 0 1.0000 Actinomycetia All All 0 1.0000 Gram Positive - All All All All 0.421 0.0325 * Gram Positive - Firmicutes Bacilli All All 0 1.0000 2 Supplemental Table 2. -

Development of Molecular Markers for Detection of Acidovorax Citrulli Strains Causing Bacterial Fruit Blotch Disease in Melon
International Journal of Molecular Sciences Article Development of Molecular Markers for Detection of Acidovorax citrulli Strains Causing Bacterial Fruit Blotch Disease in Melon Md. Rafiqul Islam, Mohammad Rashed Hossain, Hoy-Taek Kim *, Denison Michael Immanuel Jesse , Md. Abuyusuf, Hee-Jeong Jung, Jong-In Park and Ill-Sup Nou * Department of Horticulture, Sunchon National University, Suncheon, Jeonnam 57922, Korea; rafi[email protected] (M.R.I.); [email protected] (M.R.H.); [email protected] (D.M.I.J.); [email protected] (M.A.); [email protected] (H.-J.J.); [email protected] (J.-I.P.) * Correspondence: [email protected] (H.-T.K.); [email protected] (I.-S.N.); Tel.: +82-61-750-3249 (I.-S.N.) Received: 4 May 2019; Accepted: 31 May 2019; Published: 2 June 2019 Abstract: Acidovorax citrulli (A. citrulli) strains cause bacterial fruit blotch (BFB) in cucurbit crops and affect melon significantly. Numerous strains of the bacterium have been isolated from melon hosts globally. Strains that are aggressively virulent towards melon and diagnostic markers for detecting such strains are yet to be identified. Using a cross-inoculation assay, we demonstrated that two Korean strains of A. citrulli, NIHHS15-280 and KACC18782, are highly virulent towards melon but avirulent/mildly virulent to the other cucurbit crops. The whole genomes of three A. citrulli strains isolated from melon and three from watermelon were aligned, allowing the design of three primer sets (AcM13, AcM380, and AcM797) that are specific to melon host strains, from three pathogenesis-related genes. These primers successfully detected the target strain NIHHS15-280 in polymerase chain reaction (PCR) assays from a very low concentration of bacterial gDNA. -

Insights from the Genome Sequence of Acidovorax Citrulli M6, a Group I Strain of the Causal Agent of Bacterial Fruit Blotch of Cucurbits
fmicb-07-00430 April 4, 2016 Time: 15:30 # 1 ORIGINAL RESEARCH published: 06 April 2016 doi: 10.3389/fmicb.2016.00430 Insights from the Genome Sequence of Acidovorax citrulli M6, a Group I Strain of the Causal Agent of Bacterial Fruit Blotch of Cucurbits Noam Eckshtain-Levi1, Dafna Shkedy2, Michael Gershovits2, Gustavo M. Da Silva3, Dafna Tamir-Ariel1, Ron Walcott3, Tal Pupko2 and Saul Burdman1* 1 Department of Plant Pathology and Microbiology and the Otto Warburg Center for Agricultural Biotechnology, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot, Israel, 2 Department of Cell Research and Immunology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel, 3 Department of Plant Pathology, The University of Georgia, Athens, GA, USA Acidovorax citrulli is a seedborne bacterium that causes bacterial fruit blotch of cucurbit plants including watermelon and melon. A. citrulli strains can be divided into two major Edited by: groups based on DNA fingerprint analyses and biochemical properties. Group I strains Gail Preston, University of Oxford, UK have been generally isolated from non-watermelon cucurbits, while group II strains Reviewed by: are closely associated with watermelon. In the present study, we report the genome Weixing Shan, sequence of M6, a group I model A. citrulli strain, isolated from melon. We used Northwest A&F University, China comparative genome analysis to investigate differences between the genome of strain Melanie J. Filiatrault, United States Department M6 and the genome of the group II model strain AAC00-1. The draft genome sequence of Agriculture-Agricultural Research of A. -

Memorias De Avances De
0 MEMORIAS DE AVANCES DE INVESTIGACIÓN POSGRADO EN FITOSANIDAD 2019 COLEGIO DE POSTGRADUADOS CAMPUS MONTECILLO EDITORES OBDULIA LOURDES SEGURA LEON REMIGIO ANASTACIO GUZMÁN PLAZOLA MARCO ANTONIO MAGALLANES TAPIA GONZALO ESPINOSA VÁSQUEZ COMITÉ ORGANIZADOR REMIGIO ANASTACIO GUZMÁN PLAZOLA OBDULIA LOURDES SEGURA LEÓN MARCO ANTONIO MAGALLANES TAPIA GONZALO ESPINOSA VÁSQUEZ COORDINACIÓN DE DIFUSIÓN CLAUDIA CONTRERAS ORTÍZ DISEÑO DE PORTADA JORGE VALDEZ CARRASCO Montecillo, Texcoco, México, 15 de noviembre de 2019 0 AVANCES DE INVESTIGACIÓN - POSGRADO EN FITOSANIDAD 2019 PRESENTACIÓN El evento de Avances de Investigación, tuvo su origen en 1990, como una iniciativa dentro del entonces Instituto de Fitosanidad, ahora Posgrado en Fitosanidad. En 2019, se cumplen 28 años de su inicio y por primera vez; estos se unen al festejo del 60 aniversario de la creación de los Programas que le dieron su origen: Fitopatología y Entomología, en 1959. El evento de presentación de los avances de investigación del Posgrado en Fitosanidad tiene diferentes objetivos académicos, entre estos: a) que los estudiantes de maestría y doctorado próximos a graduarse como maestros o doctores en ciencias expongan, de forma oral, los trabajos de investigación que desarrollan; b) que obtengan experiencia para presentase ante un público académico experto en el área; c) favorecer la interacción académica entre las diferentes áreas de la Fitosanidad y del Colegio de Postgraduados y d) conocer los avances científicos y tecnológicos que se desarrollan en favor de la protección -

Genomic Features of Bacterial Adaptation to Plants
ARTICLES https://doi.org/10.1038/s41588-017-0012-9 Genomic features of bacterial adaptation to plants Asaf Levy 1, Isai Salas Gonzalez2,3, Maximilian Mittelviefhaus4, Scott Clingenpeel 1, Sur Herrera Paredes 2,3,15, Jiamin Miao5,16, Kunru Wang5, Giulia Devescovi6, Kyra Stillman1, Freddy Monteiro2,3, Bryan Rangel Alvarez1, Derek S. Lundberg2,3, Tse-Yuan Lu7, Sarah Lebeis8, Zhao Jin9, Meredith McDonald2,3, Andrew P. Klein2,3, Meghan E. Feltcher2,3,17, Tijana Glavina Rio1, Sarah R. Grant 2, Sharon L. Doty 10, Ruth E. Ley 11, Bingyu Zhao5, Vittorio Venturi6, Dale A. Pelletier7, Julia A. Vorholt4, Susannah G. Tringe 1,12*, Tanja Woyke 1,12* and Jeffery L. Dangl 2,3,13,14* Plants intimately associate with diverse bacteria. Plant-associated bacteria have ostensibly evolved genes that enable them to adapt to plant environments. However, the identities of such genes are mostly unknown, and their functions are poorly characterized. We sequenced 484 genomes of bacterial isolates from roots of Brassicaceae, poplar, and maize. We then com- pared 3,837 bacterial genomes to identify thousands of plant-associated gene clusters. Genomes of plant-associated bacteria encode more carbohydrate metabolism functions and fewer mobile elements than related non-plant-associated genomes do. We experimentally validated candidates from two sets of plant-associated genes: one involved in plant colonization, and the other serving in microbe–microbe competition between plant-associated bacteria. We also identified 64 plant-associated pro- tein domains that potentially mimic plant domains; some are shared with plant-associated fungi and oomycetes. This work expands the genome-based understanding of plant–microbe interactions and provides potential leads for efficient and sustain- able agriculture through microbiome engineering. -

Physiological and Genomic Features of Highly Alkaliphilic Hydrogen-Utilizing Betaproteobacteria from a Continental Serpentinizing Site
ARTICLE Received 17 Dec 2013 | Accepted 16 Apr 2014 | Published 21 May 2014 DOI: 10.1038/ncomms4900 OPEN Physiological and genomic features of highly alkaliphilic hydrogen-utilizing Betaproteobacteria from a continental serpentinizing site Shino Suzuki1, J. Gijs Kuenen2,3, Kira Schipper1,3, Suzanne van der Velde2,3, Shun’ichi Ishii1, Angela Wu1, Dimitry Y. Sorokin3,4, Aaron Tenney1, XianYing Meng5, Penny L. Morrill6, Yoichi Kamagata5, Gerard Muyzer3,7 & Kenneth H. Nealson1,2 Serpentinization, or the aqueous alteration of ultramafic rocks, results in challenging environments for life in continental sites due to the combination of extremely high pH, low salinity and lack of obvious electron acceptors and carbon sources. Nevertheless, certain Betaproteobacteria have been frequently observed in such environments. Here we describe physiological and genomic features of three related Betaproteobacterial strains isolated from highly alkaline (pH 11.6) serpentinizing springs at The Cedars, California. All three strains are obligate alkaliphiles with an optimum for growth at pH 11 and are capable of autotrophic growth with hydrogen, calcium carbonate and oxygen. The three strains exhibit differences, however, regarding the utilization of organic carbon and electron acceptors. Their global distribution and physiological, genomic and transcriptomic characteristics indicate that the strains are adapted to the alkaline and calcium-rich environments represented by the terrestrial serpentinizing ecosystems. We propose placing these strains in a new genus ‘Serpentinomonas’. 1 J. Craig Venter Institute, 4120 Torrey Pines Road, La Jolla, California 92037, USA. 2 University of Southern California, 835 W. 37th St. SHS 560, Los Angeles, California 90089, USA. 3 Delft University of Technology, Julianalaan 67, Delft, 2628BC, The Netherlands. -
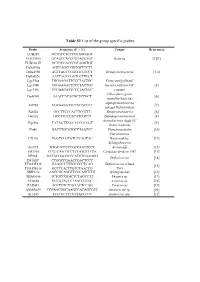
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

Delajieldii, E. Falsen (EF) Group 13, EF Group 16, and Several Clinical Isolates, with the Species Acidovorax Facilis Comb
INTERNATIONAL JOURNAL OF SYSTEMATIC BACTERIOLOGY, OCt. 1990, p. 384-398 Vol. 40, No. 4 OO20-7713/90/040384-15$02.OO/O Copyright 0 1990, International Union of Microbiological Societies Acidovorax, a New Genus for Pseudomonas facilis, Pseudomonas delaJieldii, E. Falsen (EF) Group 13, EF Group 16, and Several Clinical Isolates, with the Species Acidovorax facilis comb. nov. , Acidovorax delaJieldii comb. nov., and Acidovorax temperans sp. nov. A. WILLEMS,l E. FALSEN,2 B. POT,l E. JANTZEN,3 B. HOSTE,l P. VANDAMME,' M. GILLIS,l* K. KERSTERS,l AND J. DE LEY' Laboratorium voor Microbiologie en Microbiele Genetica, Rijksuniversiteit, B-9000 Ghent, Belgium'; Culture Collection, Department of Clinical Bacteriology, University of Goteborg, ,5413 46 Goteborg, Sweden2; and Department of Methodology, National Institute of Public Health, N-0462 Oslo, Norway3 Pseudomonas facilis and Pseudomonas delafeldii are inappropriately assigned to the genus Pseudomonas. They belong to the acidovorans rRNA complex in rRNA superfamily I11 (i.e., the beta subclass of the Proteobacteria). The taxonomic relationships of both of these species, two groups of clinical isolates (E. Falsen [EF] group 13 and EF group 16), and several unidentified or presently misnamed strains were examined by using DNA:rRNA hybridization, numerical analyses of biochemical and auxanographic features and of fatty acid patterns, polyacrylamide gel electrophoresis of cellular proteins, and DNA:DNA hybridization. These organisms form a separate group within the acidovorans rRNA complex, and we