The Nature of Chemical Hazards & Implications of GHS Applied To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446
Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION MEMORANDUM Date: March 11, 2020 SUBJECT: Registration Review Draft Risk Assessment for the Peroxy Compounds PC Code: 000595, 063201, 063604, 063607, DP Barcode: 455445, 455446 063209, 128860 Decision No: 558073, 558074 Docket No: EPA-HQ-OPP-2009-0546 Regulatory Action: Registration Review Case No: 6059, 4072, 5081 Risk Assessment Type: DRA CAS No: 7722-84-1, 79-21-0, 33734-57-5, 15630-89-4, 10058-23-8, 70693-62-8 TO: Kendall Ziner, Chemical Review Manager Rick Fehir, Ph.D., Team Lead Rose Kyprianou, Branch Chief Regulatory Management Branch (RMB) II Antimicrobials Division (7510P) Office of Pesticide Programs FROM: Andrew Byro, Ph.D., Chemist Kathryn Korthauer, Biologist Timothy Dole, Industrial Hygienist Deborah Burgin, Ph.D., DABT, Toxicologist Risk Assessment and Science Support Branch Antimicrobials Division (7510P) Office of Pesticide Programs THROUGH: Judy Facey, Ph.D., Human Health Risk Assessment Process Leader MP for JF Diana Hsieh, Ecological Risk Assessment Process Leader MP for DH Timothy Leighton, Senior Science Advisor MP for TL Laura Parsons, Associate Branch Chief Melissa Panger, Ph.D., Branch Chief Risk Assessment and Science Support Branch Antimicrobials Division (7510P) This document provides the draft human health and ecological risk assessment conducted in support of the antimicrobial use sites of the following peroxy compounds: hydrogen peroxide, peracetic acid, peroxyoctanoic acid, and sodium percarbonate. Page 1 of 74 Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 Although the peroxymonosulfate compounds were included in the peroxy compounds Final Work Plan (FWP), they will not be included in this risk assessment. -

Job Hazard Analysis
Identifying and Evaluating Hazards in Research Laboratories Guidelines developed by the Hazards Identification and Evaluation Task Force of the American Chemical Society’s Committee on Chemical Safety Copyright 2013 American Chemical Society Table of Contents FOREWORD ................................................................................................................................................... 3 ACKNOWLEDGEMENTS ................................................................................................................................. 5 Task Force Members ..................................................................................................................................... 6 1. SCOPE AND APPLICATION ..................................................................................................................... 7 2. DEFINITIONS .......................................................................................................................................... 7 3. HAZARDS IDENTIFICATION AND EVALUATION ................................................................................... 10 4. ESTABLISHING ROLES AND RESPONSIBILITIES .................................................................................... 14 5. CHOOSING AND USING A TECHNIQUE FROM THIS GUIDE ................................................................. 17 6. CHANGE CONTROL .............................................................................................................................. 19 7. ASSESSING -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Cem-Seal-SDS Sheet
Cem-Seal ICP Building Solutions Group/Pli-Dek Version No: 1.2 Issue Date: 10/26/2020 Safety Data Sheet according to OSHA HazCom Standard (2012) requirements Print Date: 10/26/2020 S.GHS.USA.EN SECTION 1 Identification Product Identifier Product name Cem-Seal Synonyms Not Available Other means of identification Not Available Recommended use of the chemical and restrictions on use Relevant identified uses Specialty floor coating Name, address, and telephone number of the chemical manufacturer, importer, or other responsible party Registered company name ICP Building Solutions Group/Pli-Dek Address 4565 W. Watkins Street Phoenix AZ Not applicable Telephone 623-435-2277 Fax Not Available Website www.ICPGROUP.com Email Not Available Emergency phone number Association / Organisation ChemTel Emergency telephone 1-800-255-3924 numbers Other emergency telephone 1-813-248-0585 numbers SECTION 2 Hazard(s) identification Classification of the substance or mixture NFPA 704 diamond Note: The hazard category numbers found in GHS classification in section 2 of this SDSs are NOT to be used to fill in the NFPA 704 diamond. Blue = Health Red = Fire Yellow = Reactivity White = Special (Oxidizer or water reactive substances) Classification Acute Aquatic Hazard Category 3 Label elements Hazard pictogram(s) Not Applicable Signal word Not Applicable Hazard statement(s) H402 Harmful to aquatic life. Hazard(s) not otherwise classified Not Applicable Precautionary statement(s) General P101 If medical advice is needed, have product container or label at hand. P102 Keep out of reach of children. Page 1 continued... Version No: 1.2 Page 2 of 8 Issue Date: 10/26/2020 Cem-Seal Print Date: 10/26/2020 Precautionary statement(s) Prevention P273 Avoid release to the environment. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -

Controlling Chemical Exposure Industrial Hygiene Fact Sheets
Controlling Chemical Exposure Industrial Hygiene Fact Sheets Concise guidance on 16 components of industrial hygiene controls New Jersey Department of Health and Senior Services Division of Epidemiology, Environmental and Occupational Health Occupational Health Service PO Box 360 Trenton, NJ 08625-0360 609-984-1863 October 2000 James E. McGreevey Clifton R. Lacy, M.D. Governor Commissioner Written by: Eileen Senn, MS, CIH Occupational Health Surveillance Program James S. Blumenstock Senior Assistant Commissioner Public Health Protection and Prevention Programs Eddy Bresnitz, MD, MS State Epidemiologist/Assistant Commissioner Division of Epidemiology, Environmental and Occupational Health Kathleen O’Leary, MS Director Occupational Health Service David Valiante, MS, CIH Acting Program Manager Occupational Health Surveillance Program Funding: This project was supported in part by a cooperative agreement from the U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health (NIOSH). Reproduction: The NJDHSS encourages the copying and distribution of all or parts of this booklet. All materials are in the public domain and may be reproduced or copied without permission. Cita- tion as to the source is appreciated. This document is available on the Internet at: www.state.nj.us/health/eoh/survweb/ihfs.pdf Citation: Senn, E., Controlling Chemical Exposure; Industrial Hygiene Fact Sheets, Trenton, NJ: New Jersey Department of Health and Senior Services, October 2000. Table of Contents Methods for Controlling -
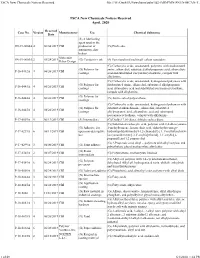
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

Job Safety Analysis
Job Safety Analysis BWC Division of Safety and Hygiene Training Center Introduction Job Safety Analysis Table of Contents Tab Page Introduction Objectives 2 Agenda 3 BWC Office Locations 4 Introduction Slides 5 PowerPoint 9 Content JSA Educational Material 25 Job Hazard Control Plan 32 Job Safety Analysis Review Checklist 36 Sample Approved JSA 39 Typical Errors on JSA 40 Sample Job Hazard Analysis 41 Downloading Materials (one-hour presentation) from Web 42 Resources Additional Resources 43 JSA Planning Sheet 45 Hazard Identification Checklist 46 Questions to Ask When Determining Controls 48 National Safety Council JSA & Directions 49 PNW/APS Safety Performance 51 OSHA Job Safety Analysis 55 JSA Forms 81 February 2008 Printed within BWC Job Safety Analysis Objectives You will learn: - A proactive approach to incident prevention and safety; - The purpose and benefits of a JSA; - Techniques for performing a JSA; - How to conduct and document a JSA; - How to analyze the results of your JSA; - How to implement your safe job procedures; - How to manage and maintain your JSA process. 2 Job Safety Analysis Agenda 8:30 am - 11:30 am - Introductions - What do you want to take away from class? - Review material in book - How to do a JSA - Video and group discussion of video afterwards 11:30 am - 12:30 pm - Lunch 1:30 pm - 3:30 pm - JSA practical exercises - Group discussion of and explanation of results of exercises: corrective actions, identifying steps, and identifying hazards. 3:30 pm - 4:30 pm - Review session - Website demos - Q&A - Evaluations 3 BWC Columbus Logan 30 W. -

Chapter 9: Chemical Hazard Information
CHEMICAL HAZARD INFORMATION CHAPTER 9 CONTENTS Overview ............................................................................................... 121 Reactive nature of chemicals ............................................................... 121 Organization of the chemical hazard information table ......................... 124 CHEMICAL HAZARD INFORMATION | Overview Many chemicals have minimal hazards, making them relatively safe to use. Others pose inherent risks and require specific precautions. Still, other chemicals must be handled with such extreme care that they are not practical or safe for school usage. This chapter provides a link to information on hazards for nearly 600 chemicals to help teachers, schools and divisions select and safely use chemicals. Schools and jurisdictions may use this information as a starting point for reviewing chemicals currently on its shelves (particularly where chemicals have accumulated over the years), and reassessing the scope and contents of chemical inventories. The information in this chapter includes numeric ratings for health, flammability and reactivity, plus supplementary comments on the scope and severity of hazards. It also includes WHMIS and storage classifications, as well as transportation hazard classes and disposal methods.This information has been compiled from the most reliable and accurate sources available at the time of writing. Inclusion of a chemical in the link listing does not signal appropriateness for school use, but is provided as preliminary information on potential concerns. Given the nature and severity of hazards involved, some of the chemicals listed are designated as not appropriate for use in schools due to safety considerations. Readers are advised to consult MSDS sheets and other current sources of more detailed information before using any of the chemicals listed in the link. Omission from this list is also not an indication of safety. -

HAZARD COMMUNICATION COMPLIANCE CHECKLIST Item Y N
HAZARD COMMUNICATION COMPLIANCE CHECKLIST Item Y N 1. Population Identification A criterion is established to determine employees that need Hazard Communication (HAZCOM) training? [The ANew Employee/Guest Orientation” form may be one method of compliance] 2. Training for identified populations a. Workers have received HAZCOM Standard Training (IND 200) [Retraining every two years is recommended. The audit criteria will be a current understanding of the Hazard Communication program and chemical safety by the employee.] b. Workers have received training on hazards specific to their area [May include “on-the- job” training, DACUMS, JTA, JSA, discussions with supervisor, tool box training, etc.] c. Workers are informed of safety requirements when new hazards are introduced into the workplace. 3. Hazard Information a. A copy of MSDSs for all chemicals used by the worker is kept in the location OR the BNL on- line MSDS system is used (http://www.esh.bnl.gov/cms/). b. Workers can demonstrate how to obtain a Material Safety Data Sheet (MSDS). c. MSDSs for chemicals, NOT acquired through Supply & Material Receiving are forwarded to the Safety & Health Services Division MSDS program (Building 129). d. Workers can demonstrate the ability to comprehend hazard information from MSDSs. e. Workers have a clear understanding of the hazards of the chemical they use (based on training and review of the MSDS). 4. Hazard Recognition and Control a. The supervisor (or cognizant individual) conducts a review prior to the use of chemicals to determine the appropriate protective measures. b. Workers follow appropriate protective measures established by their supervisor. 1. Hoods, vents or other engineering controls are used as necessary. -

Hazard Communication and the Globally
HAZARD COMMUNICATION AND THE GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELING OF CHEMICALS HAZARD COMMUNICATION AND THE GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELING OF CHEMICALS Contents Labels & Pictograms OSHA Brief: Hazard Communication Standards: Labels and Pictograms ..........3 OSHA Quick Card: Hazard Communication Standard Pictogram ................... 12 OSHA Quick Card: Hazard Communication Standard Labels ......................... 13 Safety Data Sheets OSHA Quick Card: Hazard Communication Standard Safety Data Sheets ...... 14 OSHA Brief: Hazard Communication Standard: Safety Data Sheets .............. 16 Material Safety Data Sheet: Clorox (old format) .......................................... 23 Safety Data Sheet: OxyChem (new format) ............................................... 24 Safety Data Sheet: Tedia (for Practice Exercise) ......................................... 34 Hazard Communication — SDS Practice Exercises ....................................... 41 HAZARD COMMUNICATION AND THE GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELING OF CHEMICALS 1 2 HAZARD COMMUNICATION AND THE GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELING OF CHEMICALS BRIEF Hazard Communication Standard: Labels and Pictograms OSHA has adopted new hazardous chemical standard also requires the use of a 16-section labeling requirements as a part of its recent safety data sheet format, which provides revision of the Hazard Communication detailed information regarding the chemical. Standard, 29 CFR 1910.1200 (HCS), bringing There is a separate OSHA Brief on SDSs it into alignment with the United Nations’ that provides information on the new SDS Globally Harmonized System of Classification requirements. and Labelling of Chemicals (GHS). These changes will help ensure improved quality and All hazardous chemicals shipped after June 1, consistency in the classification and labeling 2015, must be labeled with specified elements of all chemicals, and will also enhance worker including pictograms, signal words and hazard comprehension.