Distinct Parietal and Temporal Connectivity Profiles of Ventrolateral Frontal Areas Involved in Language Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
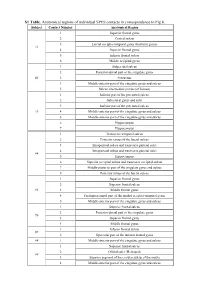
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

The Role of the Superior Temporal Sulcus and the Mirror Neuron System in Imitation
r Human Brain Mapping 31:1316–1326 (2010) r The Role of the Superior Temporal Sulcus and the Mirror Neuron System in Imitation Pascal Molenberghs,* Christopher Brander, Jason B. Mattingley, and Ross Cunnington The University of Queensland, Queensland Brain Institute & School of Psychology, St Lucia, Queensland, Australia r r Abstract: It has been suggested that in humans the mirror neuron system provides a neural substrate for imitation behaviour, but the relative contributions of different brain regions to the imitation of manual actions is still a matter of debate. To investigate the role of the mirror neuron system in imita- tion we used fMRI to examine patterns of neural activity under four different conditions: passive ob- servation of a pantomimed action (e.g., hammering a nail); (2) imitation of an observed action; (3) execution of an action in response to a word cue; and (4) self-selected execution of an action. A net- work of cortical areas, including the left supramarginal gyrus, left superior parietal lobule, left dorsal premotor area and bilateral superior temporal sulcus (STS), was significantly active across all four con- ditions. Crucially, within this network the STS bilaterally was the only region in which activity was significantly greater for action imitation than for the passive observation and execution conditions. We suggest that the role of the STS in imitation is not merely to passively register observed biological motion, but rather to actively represent visuomotor correspondences between one’s own actions and the actions of others. Hum Brain Mapp 31:1316–1326, 2010. VC 2010 Wiley-Liss, Inc. Key words: fMRI; imitation; mirror neuron system r r INTRODUCTION ror neurons are visuomotor neurons that fire both when an action is performed and when a similar or identical Motor imitation involves observing the action of another action is passively observed [Rizzolatti and Craighero, individual and matching one’s own movements to those 2004]. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Stimulus Arousal Drives Amygdalar Responses to Emotional
www.nature.com/scientificreports OPEN Stimulus arousal drives amygdalar responses to emotional expressions across sensory modalities Huiyan Lin1,2,4*, Miriam Müller-Bardorf2,4, Bettina Gathmann2,4, Jaqueline Brieke2, Martin Mothes-Lasch2, Maximilian Bruchmann2, Wolfgang H. R. Miltner3 & Thomas Straube2 The factors that drive amygdalar responses to emotionally signifcant stimuli are still a matter of debate – particularly the proneness of the amygdala to respond to negatively-valenced stimuli has been discussed controversially. Furthermore, it is uncertain whether the amygdala responds in a modality-general fashion or whether modality-specifc idiosyncrasies exist. Therefore, the present functional magnetic resonance imaging (fMRI) study systematically investigated amygdalar responding to stimulus valence and arousal of emotional expressions across visual and auditory modalities. During scanning, participants performed a gender judgment task while prosodic and facial emotional expressions were presented. The stimuli varied in stimulus valence and arousal by including neutral, happy and angry expressions of high and low emotional intensity. Results demonstrate amygdalar activation as a function of stimulus arousal and accordingly associated emotional intensity regardless of stimulus valence. Furthermore, arousal-driven amygdalar responding did not depend on the visual and auditory modalities of emotional expressions. Thus, the current results are consistent with the notion that the amygdala codes general stimulus relevance across visual -

Surgical Anatomy of the Insula Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura
Surgical Anatomy of the Insula Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura To cite this version: Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura. Surgical Anatomy of the Insula. Mehmet Turgut; Canan Yurttaş; R. Shane Tubbs. Island of Reil (Insula) in the Human Brain. Anatomical, Functional, Clinical and Surgical Aspects, 19, Springer, pp.23-37, 2018, 978-3-319-75467-3. 10.1007/978-3-319-75468-0_3. hal-02539550 HAL Id: hal-02539550 https://hal.archives-ouvertes.fr/hal-02539550 Submitted on 15 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Surgical Anatomy of the insula Christophe Destrieux1, 2, Igor Lima Maldonado3, Louis-Marie Terrier1, 2, Ilyess Zemmoura1, 2 1 : Université François-Rabelais de Tours, Inserm, Imagerie et cerveau UMR 930, Tours, France 2 : CHRU de Tours, Service de Neurochirurgie , Tours, France 3 : Universidade Federal da Bahia, Laboratório de Anatomia e Dissecção, Departamento de Biomorfologia - Instituto de Ciências da Saúde, Salvador-Bahia, Brazil 1. Abstract The insula was for a long time considered as one of the most challenging areas of the brain. This is mainly related to its location, deep and medial to the fronto-parietal, temporal and fronto-orbital opercula. -

Translingual Neural Stimulation with the Portable Neuromodulation
Translingual Neural Stimulation With the Portable Neuromodulation Stimulator (PoNS®) Induces Structural Changes Leading to Functional Recovery In Patients With Mild-To-Moderate Traumatic Brain Injury Authors: Jiancheng Hou,1 Arman Kulkarni,2 Neelima Tellapragada,1 Veena Nair,1 Yuri Danilov,3 Kurt Kaczmarek,3 Beth Meyerand,2 Mitchell Tyler,2,3 *Vivek Prabhakaran1 1. Department of Radiology, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin, USA 2. Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, Wisconsin, USA 3. Department of Kinesiology, University of Wisconsin-Madison, Madison, Wisconsin, USA *Correspondence to [email protected] Disclosure: Dr Tyler, Dr Danilov, and Dr Kaczmarek are co-founders of Advanced Neurorehabilitation, LLC, which holds the intellectual property rights to the PoNS® technology. Dr Tyler is a board member of NeuroHabilitation Corporation, a wholly- owned subsidiary of Helius Medical Technologies, and owns stock in the corporation. The other authors have declared no conflicts of interest. Acknowledgements: Professional medical writing and editorial assistance were provided by Kelly M. Fahrbach, Ashfield Healthcare Communications, part of UDG Healthcare plc, funded by Helius Medical Technologies. Dr Tyler, Dr Kaczmarek, Dr Danilov, Dr Hou, and Dr Prabhakaran were being supported by NHC-TBI-PoNS-RT001. Dr Hou, Dr Kulkarni, Dr Nair, Dr Tellapragada, and Dr Prabhakaran were being supported by R01AI138647. Dr Hou and Dr Prabhakaran were being supported by P01AI132132, R01NS105646. Dr Kulkarni was being supported by the Clinical & Translational Science Award programme of the National Center for Research Resources, NCATS grant 1UL1RR025011. Dr Meyerand, Dr Prabhakaran, Dr Nair was being supported by U01NS093650. -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Dissociable Roles of Mid-Dorsolateral Prefrontal and Anterior Inferotemporal Cortex in Visual Working Memory
The Journal of Neuroscience, October 1, 2000, 20(19):7496–7503 Dissociable Roles of Mid-Dorsolateral Prefrontal and Anterior Inferotemporal Cortex in Visual Working Memory Michael Petrides Montreal Neurological Institute and Department of Psychology, McGill University, Montreal, Quebec H3A 2B4, Canada Functional neuroimaging in human subjects and studies of mon- anterior inferotemporal lesions. This demonstration of a double keys with lesions limited to the mid-dorsolateral (MDL) prefrontal dissociation between the effects of these two lesions provides cortex have shown that this specific region of the prefrontal strong evidence that the role of the mid-dorsolateral prefrontal cortex is involved in visual working memory, although its precise cortex in visual working memory does not lie in the maintenance role remains a matter of debate. The present study compared the of information per se, but rather in the executive process of effect on visual working memory of lesions restricted to the monitoring this information. In addition, the present study dem- mid-dorsolateral prefrontal cortex of the monkey with that of onstrated that lesions limited to area 9, which constitutes the lesions to the anterior inferotemporal cortex, a region of the superior part of the mid-dorsolateral prefrontal region, give rise to temporal cortex specialized for visual memory. Increasing the a mild impairment in the monitoring of information, whereas delay during which information had to be maintained in visual lesions of the complete mid-dorsolateral prefrontal region yield a working memory impaired performance after lesions of the an- very severe impairment. terior inferotemporal cortex, but not after mid-dorsolateral pre- frontal lesions. -

Down but Not out in Posterior Cingulate Cortex: Deactivation Yet Functional Coupling with Prefrontal Cortex During Demanding Semantic Cognition
NeuroImage 141 (2016) 366–377 Contents lists available at ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Down but not out in posterior cingulate cortex: Deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition Katya Krieger-Redwood ⁎, Elizabeth Jefferies, Theodoros Karapanagiotidis, Robert Seymour, Adonany Nunes, Jit Wei Aaron Ang, Vierra Majernikova, Giovanna Mollo, Jonathan Smallwood Department of Psychology/York Neuroimaging Centre, University of York, Heslington, York, United Kingdom article info abstract Article history: The posterior cingulate cortex (pCC) often deactivates during complex tasks, and at rest is often only weakly cor- Received 30 March 2016 related with regions that play a general role in the control of cognition. These observations led to the hypothesis Accepted 29 July 2016 that pCC contributes to automatic aspects of memory retrieval and cognition. Recent work, however, has sug- Available online 30 July 2016 gested that the pCC may support both automatic and controlled forms of memory processing and may do so by changing its communication with regions that are important in the control of cognition across multiple do- Keywords: mains. The current study examined these alternative views by characterising the functional coupling of the Posterior cingulate cortex Dorsolateral prefrontal cortex pCC in easy semantic decisions (based on strong global associations) and in harder semantic tasks (matching Semantic control words on the basis of specific non-dominant features). Increasingly difficult semantic decisions led to the expect- Executive ed pattern of deactivation in the pCC; however, psychophysiological interaction analysis revealed that, under Rest these conditions, the pCC exhibited greater connectivity with dorsolateral prefrontal cortex (PFC), relative to Connectivity both easier semantic decisions and to a period of rest. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

Keeping Order in the Brain: the Supramarginal Gyrus and Serial Order in Short-Term Memory
cortex 119 (2019) 89e99 Available online at www.sciencedirect.com ScienceDirect Journal homepage: www.elsevier.com/locate/cortex Research Report Keeping order in the brain: The supramarginal gyrus and serial order in short-term memory Giacomo Guidalia,b, Alberto Pisonia, Nadia Bologninia,c and * Costanza Papagnoa,d, a Department of Psychology & Milan Center for Neuroscience - NeuroMI, University of Milano-Bicocca, Milan, Italy b Ph.D. program in Neuroscience, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy c Laboratory of Neuropsychology, IRCCS Istituto Auxologico Italiano, Milan, Italy d CIMeC & CeRiN, University of Trento, Rovereto, Italy article info abstract Article history: A wide range of human activities are performed sequentially in few seconds. We need to Received 13 December 2018 maintain a correct temporal order of words in language, movements in actions, directions Reviewed 5 February 2019 in navigation, etc. Therefore, it is plausible, in a more economical perspective, that our Revised 22 February 2019 brain is equipped with a dedicated mechanism for storing a temporal sequence for a short Accepted 10 April 2019 time. To investigate it, we run four TMS experiments, in which participants performed Action editor Eric Wassermann different short-term memory tasks, i.e., three (verbal, spatial, motor) requiring mainte- Published online 20 April 2019 nance of an ordered sequence and one (visual) of a static pattern. We demonstrated, for the first time, that the left supramarginal gyrus is one of the key nodes of the STM network Keywords: involved in retaining an abstract representation of serial order information, independently Short-term memory from the content information, namely the nature of the item to be remembered, which Serial order instead is stored separately.