Differential Integrin Adhesome Expression Defines Human Natural
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

List of Genes Used in Cell Type Enrichment Analysis
List of genes used in cell type enrichment analysis Metagene Cell type Immunity ADAM28 Activated B cell Adaptive CD180 Activated B cell Adaptive CD79B Activated B cell Adaptive BLK Activated B cell Adaptive CD19 Activated B cell Adaptive MS4A1 Activated B cell Adaptive TNFRSF17 Activated B cell Adaptive IGHM Activated B cell Adaptive GNG7 Activated B cell Adaptive MICAL3 Activated B cell Adaptive SPIB Activated B cell Adaptive HLA-DOB Activated B cell Adaptive IGKC Activated B cell Adaptive PNOC Activated B cell Adaptive FCRL2 Activated B cell Adaptive BACH2 Activated B cell Adaptive CR2 Activated B cell Adaptive TCL1A Activated B cell Adaptive AKNA Activated B cell Adaptive ARHGAP25 Activated B cell Adaptive CCL21 Activated B cell Adaptive CD27 Activated B cell Adaptive CD38 Activated B cell Adaptive CLEC17A Activated B cell Adaptive CLEC9A Activated B cell Adaptive CLECL1 Activated B cell Adaptive AIM2 Activated CD4 T cell Adaptive BIRC3 Activated CD4 T cell Adaptive BRIP1 Activated CD4 T cell Adaptive CCL20 Activated CD4 T cell Adaptive CCL4 Activated CD4 T cell Adaptive CCL5 Activated CD4 T cell Adaptive CCNB1 Activated CD4 T cell Adaptive CCR7 Activated CD4 T cell Adaptive DUSP2 Activated CD4 T cell Adaptive ESCO2 Activated CD4 T cell Adaptive ETS1 Activated CD4 T cell Adaptive EXO1 Activated CD4 T cell Adaptive EXOC6 Activated CD4 T cell Adaptive IARS Activated CD4 T cell Adaptive ITK Activated CD4 T cell Adaptive KIF11 Activated CD4 T cell Adaptive KNTC1 Activated CD4 T cell Adaptive NUF2 Activated CD4 T cell Adaptive PRC1 Activated -

Markers of T Cell Senescence in Humans
International Journal of Molecular Sciences Review Markers of T Cell Senescence in Humans Weili Xu 1,2 and Anis Larbi 1,2,3,4,5,* 1 Biology of Aging Program and Immunomonitoring Platform, Singapore Immunology Network (SIgN), Agency for Science Technology and Research (A*STAR), Immunos Building, Biopolis, Singapore 138648, Singapore; [email protected] 2 School of Biological Sciences, Nanyang Technological University, Singapore 637551, Singapore 3 Department of Microbiology, National University of Singapore, Singapore 117597, Singapore 4 Department of Geriatrics, Faculty of Medicine, University of Sherbrooke, Sherbrooke, QC J1K 2R1, Canada 5 Faculty of Sciences, University ElManar, Tunis 1068, Tunisia * Correspondence: [email protected]; Tel.: +65-6407-0412 Received: 31 May 2017; Accepted: 26 July 2017; Published: 10 August 2017 Abstract: Many countries are facing the aging of their population, and many more will face a similar obstacle in the near future, which could be a burden to many healthcare systems. Increased susceptibility to infections, cardiovascular and neurodegenerative disease, cancer as well as reduced efficacy of vaccination are important matters for researchers in the field of aging. As older adults show higher prevalence for a variety of diseases, this also implies higher risk of complications, including nosocomial infections, slower recovery and sequels that may reduce the autonomy and overall quality of life of older adults. The age-related effects on the immune system termed as “immunosenescence” can be exemplified by the reported hypo-responsiveness to influenza vaccination of the elderly. T cells, which belong to the adaptive arm of the immune system, have been extensively studied and the knowledge gathered enables a better understanding of how the immune system may be affected after acute/chronic infections and how this matters in the long run. -

Expression Tissue
The Journal of Immunology Killer Cell Ig-Like Receptor and Leukocyte Ig-Like Receptor Transgenic Mice Exhibit Tissue- and Cell-Specific Transgene Expression1 Danny Belkin,* Michaela Torkar,* Chiwen Chang,* Roland Barten,* Mauro Tolaini,† Anja Haude,* Rachel Allen,* Michael J. Wilson,* Dimitris Kioussis,† and John Trowsdale2* To generate an experimental model for exploring the function, expression pattern, and developmental regulation of human Ig-like activating and inhibitory receptors, we have generated transgenic mice using two human genomic clones: 52N12 (a 150-Kb clone encompassing the leukocyte Ig-like receptor (LILR)B1 (ILT2), LILRB4 (ILT3), and LILRA1 (LIR6) genes) and 1060P11 (a 160-Kb clone that contains ten killer cell Ig-like receptor (KIR) genes). Both the KIR and LILR families are encoded within the leukocyte receptor complex, and are involved in immune modulation. We have also produced a novel mAb to LILRA1 to facilitate expression studies. The LILR transgenes were expressed in a similar, but not identical, pattern to that observed in humans: LILRB1 was expressed in B cells, most NK cells, and a small number of T cells; LILRB4 was expressed in a B cell subset; and LILRA1 was found on a ring of cells surrounding B cell areas on spleen sections, consistent with other data showing monocyte/macrophage expression. KIR transgenic mice showed KIR2DL2 expression on a subset of NK cells and T cells, similar to the pattern seen in humans, and expression of KIR2DL4, KIR3DS1, and KIR2DL5 by splenic NK cells. These observations indicate that linked regulatory elements within the genomic clones are sufficient to allow appropriate expression of KIRs in mice, and illustrate that the presence of the natural ligands for these receptors, in the form of human MHC class I proteins, is not necessary for the expression of the KIRs observed in these mice. -

Human Β‑1,3‑Glucuronyltransferase 1/B3GAT1 Antibody Monoclonal Mouse Igg2a Clone # 1002707 Catalog Number: MAB8560
Human β‑1,3‑Glucuronyltransferase 1/B3GAT1 Antibody Monoclonal Mouse IgG2A Clone # 1002707 Catalog Number: MAB8560 DESCRIPTION Species Reactivity Human Specificity Detects human β-1,3-Glucuronyltransferase 1/B3GAT1 in direct ELISAs. Source Monoclonal Mouse IgG2A Clone # 1002707 Purification Protein A or G purified from hybridoma culture supernatant Immunogen Chinese Hamster Ovary cell line, CHO-derived human β‑1,3‑Glucuronyltransferase 1/B3GAT1 His25-Ile334 Accession # Q9P2W7 Formulation Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. See Certificate of Analysis for details. *Small pack size (-SP) is supplied either lyophilized or as a 0.2 μm filtered solution in PBS. APPLICATIONS Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website. Recommended Sample Concentration Immunohistochemistry 5-25 µg/mL See Below DATA Immunohistochemistry β-1,3-Glucuronyltransferase 1/B3GAT1 in Human Brain. β-1,3-Glucuronyltransferase 1/B3GAT1 was detected in immersion fixed paraffin-embedded sections of human brain (cortex) using Mouse Anti-Human β-1,3- Glucuronyltransferase 1/B3GAT1 Monoclonal Antibody (Catalog # MAB8560) at 5 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC001). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm in neurons. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents. PREPARATION AND STORAGE Reconstitution Reconstitute at 0.5 mg/mL in sterile PBS. -
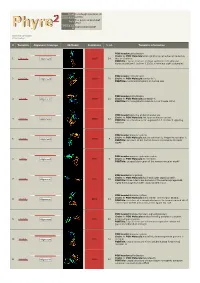
Phyre 2 Results for A1YIY0
Email [email protected] Description A1YIY0 Tue Jul 30 13:19:15 BST Date 2013 Unique Job 1035bc4b501530df ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:cell adhesion Chain: A: PDB Molecule:down syndrome cell adhesion molecule 1 c3dmkA_ Alignment 100.0 14 (dscam) isoform PDBTitle: crystal structure of down syndrome cell adhesion molecule (dscam)2 isoform 1.30.30, n-terminal eight ig domains PDB header:cell adhesion 2 c2om5A_ Alignment 100.0 20 Chain: A: PDB Molecule:contactin 2; PDBTitle: n-terminal fragment of human tax1 PDB header:cell adhesion 3 c3jxaA_ Alignment 100.0 21 Chain: A: PDB Molecule:contactin 4; PDBTitle: immunoglobulin domains 1-4 of mouse cntn4 PDB header:signaling protein/transferase Chain: A: PDB Molecule:tek tyrosine kinase variant; 4 c4k0vA_ 100.0 12 Alignment PDBTitle: structural basis for angiopoietin-1 mediated signaling initiation PDB header:immune system Chain: A: PDB Molecule:natural cytotoxicity triggering receptor 1; 5 c1p6fA_ 100.0 9 Alignment PDBTitle: structure of the human natural cytotoxicity receptor nkp46 PDB header:immune system/receptor 6 c1ollA_ Alignment 99.9 9 Chain: A: PDB Molecule:nk receptor; PDBTitle: extracellular region of the human receptor nkp46 PDB header:viral protein Chain: A: PDB Molecule:hoc head outer capsid protein; 7 c3shsA_ 99.9 18 Alignment PDBTitle: three n-terminal domains of the bacteriophage rb49 highly immunogenic2 outer capsid protein (hoc) PDB header:immune system Chain: E: PDB Molecule:natural -

Flow Reagents Single Color Antibodies CD Chart
CD CHART CD N° Alternative Name CD N° Alternative Name CD N° Alternative Name Beckman Coulter Clone Beckman Coulter Clone Beckman Coulter Clone T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells CD1a T6, R4, HTA1 Act p n n p n n S l CD99 MIC2 gene product, E2 p p p CD223 LAG-3 (Lymphocyte activation gene 3) Act n Act p n CD1b R1 Act p n n p n n S CD99R restricted CD99 p p CD224 GGT (γ-glutamyl transferase) p p p p p p CD1c R7, M241 Act S n n p n n S l CD100 SEMA4D (semaphorin 4D) p Low p p p n n CD225 Leu13, interferon induced transmembrane protein 1 (IFITM1). p p p p p CD1d R3 Act S n n Low n n S Intest CD101 V7, P126 Act n p n p n n p CD226 DNAM-1, PTA-1 Act n Act Act Act n p n CD1e R2 n n n n S CD102 ICAM-2 (intercellular adhesion molecule-2) p p n p Folli p CD227 MUC1, mucin 1, episialin, PUM, PEM, EMA, DF3, H23 Act p CD2 T11; Tp50; sheep red blood cell (SRBC) receptor; LFA-2 p S n p n n l CD103 HML-1 (human mucosal lymphocytes antigen 1), integrin aE chain S n n n n n n n l CD228 Melanotransferrin (MT), p97 p p CD3 T3, CD3 complex p n n n n n n n n n l CD104 integrin b4 chain; TSP-1180 n n n n n n n p p CD229 Ly9, T-lymphocyte surface antigen p p n p n -

Detection of Pro Angiogenic and Inflammatory Biomarkers in Patients With
www.nature.com/scientificreports OPEN Detection of pro angiogenic and infammatory biomarkers in patients with CKD Diana Jalal1,2,3*, Bridget Sanford4, Brandon Renner5, Patrick Ten Eyck6, Jennifer Laskowski5, James Cooper5, Mingyao Sun1, Yousef Zakharia7, Douglas Spitz7,9, Ayotunde Dokun8, Massimo Attanasio1, Kenneth Jones10 & Joshua M. Thurman5 Cardiovascular disease (CVD) is the most common cause of death in patients with native and post-transplant chronic kidney disease (CKD). To identify new biomarkers of vascular injury and infammation, we analyzed the proteome of plasma and circulating extracellular vesicles (EVs) in native and post-transplant CKD patients utilizing an aptamer-based assay. Proteins of angiogenesis were signifcantly higher in native and post-transplant CKD patients versus healthy controls. Ingenuity pathway analysis (IPA) indicated Ephrin receptor signaling, serine biosynthesis, and transforming growth factor-β as the top pathways activated in both CKD groups. Pro-infammatory proteins were signifcantly higher only in the EVs of native CKD patients. IPA indicated acute phase response signaling, insulin-like growth factor-1, tumor necrosis factor-α, and interleukin-6 pathway activation. These data indicate that pathways of angiogenesis and infammation are activated in CKD patients’ plasma and EVs, respectively. The pathways common in both native and post-transplant CKD may signal similar mechanisms of CVD. Approximately one in 10 individuals has chronic kidney disease (CKD) rendering CKD one of the most common diseases worldwide1. CKD is associated with a high burden of morbidity in the form of end stage kidney disease (ESKD) requiring dialysis or transplantation 2. Furthermore, patients with CKD are at signifcantly increased risk of death from cardiovascular disease (CVD)3,4. -

Snipa Snpcard
SNiPAcard Block annotations Block info genomic range chr19:55,117,749-55,168,602 block size 50,854 bp variant count 74 variants Basic features Conservation/deleteriousness Linked genes μ = -0.557 [-4.065 – AC009892.10 , AC009892.5 , AC009892.9 , AC245036.1 , AC245036.2 , phyloP 2.368] gene(s) hit or close-by AC245036.3 , AC245036.4 , AC245036.5 , AC245036.6 , LILRA1 , LILRB1 , LILRB4 , MIR8061 , VN1R105P phastCons μ = 0.059 [0 – 0.633] eQTL gene(s) CTB-83J4.2 , LILRA1 , LILRA2 , LILRB2 , LILRB5 , LILRP1 μ = -0.651 [-4.69 – 2.07] AC008984.5 , AC008984.5 , AC008984.6 , AC008984.6 , AC008984.7 , AC008984.7 , AC009892.10 , AC009892.10 , AC009892.2 , AC009892.2 , AC009892.5 , AC010518.3 , AC010518.3 , AC011515.2 , AC011515.2 , AC012314.19 , AC012314.19 , FCAR , FCAR , FCAR , FCAR , FCAR , FCAR , FCAR , FCAR , FCAR , FCAR , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL1 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL3 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , KIR2DL4 , -

(P -Value<0.05, Fold Change≥1.4), 4 Vs. 0 Gy Irradiation
Table S1: Significant differentially expressed genes (P -Value<0.05, Fold Change≥1.4), 4 vs. 0 Gy irradiation Genbank Fold Change P -Value Gene Symbol Description Accession Q9F8M7_CARHY (Q9F8M7) DTDP-glucose 4,6-dehydratase (Fragment), partial (9%) 6.70 0.017399678 THC2699065 [THC2719287] 5.53 0.003379195 BC013657 BC013657 Homo sapiens cDNA clone IMAGE:4152983, partial cds. [BC013657] 5.10 0.024641735 THC2750781 Ciliary dynein heavy chain 5 (Axonemal beta dynein heavy chain 5) (HL1). 4.07 0.04353262 DNAH5 [Source:Uniprot/SWISSPROT;Acc:Q8TE73] [ENST00000382416] 3.81 0.002855909 NM_145263 SPATA18 Homo sapiens spermatogenesis associated 18 homolog (rat) (SPATA18), mRNA [NM_145263] AA418814 zw01a02.s1 Soares_NhHMPu_S1 Homo sapiens cDNA clone IMAGE:767978 3', 3.69 0.03203913 AA418814 AA418814 mRNA sequence [AA418814] AL356953 leucine-rich repeat-containing G protein-coupled receptor 6 {Homo sapiens} (exp=0; 3.63 0.0277936 THC2705989 wgp=1; cg=0), partial (4%) [THC2752981] AA484677 ne64a07.s1 NCI_CGAP_Alv1 Homo sapiens cDNA clone IMAGE:909012, mRNA 3.63 0.027098073 AA484677 AA484677 sequence [AA484677] oe06h09.s1 NCI_CGAP_Ov2 Homo sapiens cDNA clone IMAGE:1385153, mRNA sequence 3.48 0.04468495 AA837799 AA837799 [AA837799] Homo sapiens hypothetical protein LOC340109, mRNA (cDNA clone IMAGE:5578073), partial 3.27 0.031178378 BC039509 LOC643401 cds. [BC039509] Homo sapiens Fas (TNF receptor superfamily, member 6) (FAS), transcript variant 1, mRNA 3.24 0.022156298 NM_000043 FAS [NM_000043] 3.20 0.021043295 A_32_P125056 BF803942 CM2-CI0135-021100-477-g08 CI0135 Homo sapiens cDNA, mRNA sequence 3.04 0.043389246 BF803942 BF803942 [BF803942] 3.03 0.002430239 NM_015920 RPS27L Homo sapiens ribosomal protein S27-like (RPS27L), mRNA [NM_015920] Homo sapiens tumor necrosis factor receptor superfamily, member 10c, decoy without an 2.98 0.021202829 NM_003841 TNFRSF10C intracellular domain (TNFRSF10C), mRNA [NM_003841] 2.97 0.03243901 AB002384 C6orf32 Homo sapiens mRNA for KIAA0386 gene, partial cds. -

Crystal Structure of the HLA-Cw3 Allotype-Specific Killer Cell Inhibitory Receptor KIR2DL2
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3864–3869, March 1999 Immunology Crystal structure of the HLA-Cw3 allotype-specific killer cell inhibitory receptor KIR2DL2 GREG A. SNYDER*, ANDREW G. BROOKS†, AND PETER D. SUN‡§ *Department of Biochemistry, Molecular Biology, and Cell Biology, 2153 Sheridan, O. T. Hogan Hall, Room 2-100, Northwestern University, Evanston, IL 60208; †Department of Microbiology and Immunology, University of Melbourne, Royal Parade, Parkville, 3052 Victoria, Australia; and ‡Structural Biology Section, Office of the Scientific Director, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 12441 Parklawn Drive, Rockville, MD 20852 Edited by David R. Davies, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, and approved January 19, 1999 (received for review November 11, 1998) ABSTRACT Killer cell inhibitory receptors (KIR) protect differ primarily in the transmembrane and the intracellular class I HLAs expressing target cells from natural killer (NK) regions. KIR2DL molecules are inhibitory receptors possess- cell-mediated lysis. To understand the molecular basis of this ing intracellular immunoreceptor tyrosine-based inhibitory receptor-ligand recognition, we have crystallized the extra- motifs (ITIM), whereas KIR2DS molecules appear to be cellular ligand-binding domains of KIR2DL2, a member of the noninhibitory receptors with shorter intracellular tails that Ig superfamily receptors that recognize HLA-Cw1, 3, 7, and 8 interact with an immunoreceptor tyrosine-based activation allotypes. The structure was determined in two different motif (ITAM) containing signaling component DAP12 (11). crystal forms, an orthorhombic P212121 and a trigonal P3221 KIR3D members have three Ig domains (known as D0, D1, space group, to resolutions of 3.0 and 2.9 Å, respectively. -

Cytolytic Activity of NK Cell Clones Against Acute Childhood Precursor
Bone Marrow Transplantation (2009) 43, 875–881 & 2009 Macmillan Publishers Limited All rights reserved 0268-3369/09 $32.00 www.nature.com/bmt ORIGINAL ARTICLE Cytolytic activity of NK cell clones against acute childhood precursor-B-cell leukaemia is influenced by HLA class I expression on blasts and the differential KIR phenotype of NK clones T Feuchtinger1, M Pfeiffer1, A Pfaffle1, H-M Teltschik1, D Wernet2, M Schumm1, R Lotfi2, R Handgretinger1 and P Lang1 1Department of Paediatric Haematology/Oncology, University Children’s Hospital, Tu¨bingen, Germany and 2Institute of Transfusion Medicine, Eberhard Karls University, Tu¨bingen, Germany Relapse after allo-SCT in patients with acute leukaemia Introduction remains a major problem. A beneficial impact of alloreactive natural killer (NK) cells has been reported Stem cell transplantation from matched or partially for myeloid malignancies, but has been questionable for matched unrelated donors as well as mismatched related B-lineage ALL. We analysed lysis of primary paediatric donors has become an established procedure for the precursor-B-ALL blasts by 285 NK cell clones to treatment of children with high risk and relapsed leukae- investigate whether HLA class I expression on the blasts mia.1–3 GVHD-related mortality has been reduced signifi- and phenotypic killer cell Ig-like receptor (KIR) expres- cantly by T-cell depletion, but relapse still remains the sion on NK cells affect the lytic activity against ALL major problem after successful transplantation.4,5 In blasts. Precursor-B-ALL blasts with low HLA-I expres- contrast to myeloid malignancies, controversial effects of sion were lysed by a majority (79%) of NK cell clones, T-cell depletion on relapse rate in ALL have been whereas those with high HLA-I expression showed low reported.1,3,6,7 As normal numbers of NK cells reconstitute susceptibility to NK clones independent of their KIR from donor progenitors within a few weeks after T-cell expression patterns.