High-Dimensional Analysis Reveals a Distinct Population of Adaptive-Like Tissue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Killer-Like Receptors and GPR56 Progressive Expression Defines Cytokine Production of Human CD4+ Memory T Cells
ARTICLE https://doi.org/10.1038/s41467-019-10018-1 OPEN Killer-like receptors and GPR56 progressive expression defines cytokine production of human CD4+ memory T cells Kim-Long Truong1,7, Stephan Schlickeiser1,2,7, Katrin Vogt1, David Boës1, Katarina Stanko1, Christine Appelt1, Mathias Streitz1, Gerald Grütz1,2, Nadja Stobutzki1, Christian Meisel1, Christina Iwert1, Stefan Tomiuk3, Julia K. Polansky2,4, Andreas Pascher5, Nina Babel2,6, Ulrik Stervbo 6, Igor Sauer 5, Undine Gerlach5 & Birgit Sawitzki1,2 1234567890():,; All memory T cells mount an accelerated response on antigen reencounter, but significant functional heterogeneity is present within the respective memory T-cell subsets as defined by CCR7 and CD45RA expression, thereby warranting further stratification. Here we show that several surface markers, including KLRB1, KLRG1, GPR56, and KLRF1, help define low, high, or exhausted cytokine producers within human peripheral and intrahepatic CD4+ memory T-cell populations. Highest simultaneous production of TNF and IFN-γ is observed in KLRB1+KLRG1+GPR56+ CD4 T cells. By contrast, KLRF1 expression is associated with T-cell exhaustion and reduced TNF/IFN-γ production. Lastly, TCRβ repertoire analysis and in vitro differentiation support a regulated, progressive expression for these markers during CD4+ memory T-cell differentiation. Our results thus help refine the classification of human memory T cells to provide insights on inflammatory disease progression and immunotherapy development. 1 Institute of Medical Immunology, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin and Berlin Institute of Health, 13353 Berlin, Germany. 2 Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, 13353 Berlin, Germany. 3 Milteny Biotec GmbH, 51429 Bergisch Gladbach, Germany. -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Tools for Cell Therapy and Immunoregulation
RnDSy-lu-2945 Tools for Cell Therapy and Immunoregulation Target Cell TIM-4 SLAM/CD150 BTNL8 PD-L2/B7-DC B7-H1/PD-L1 (Human) Unknown PD-1 B7-1/CD80 TIM-1 SLAM/CD150 Receptor TIM Family SLAM Family Butyrophilins B7/CD28 Families T Cell Multiple Co-Signaling Molecules Co-stimulatory Co-inhibitory Ig Superfamily Regulate T Cell Activation Target Cell T Cell Target Cell T Cell B7-1/CD80 B7-H1/PD-L1 T cell activation requires two signals: 1) recognition of the antigenic peptide/ B7-1/CD80 B7-2/CD86 CTLA-4 major histocompatibility complex (MHC) by the T cell receptor (TCR) and 2) CD28 antigen-independent co-stimulation induced by interactions between B7-2/CD86 B7-H1/PD-L1 B7-1/CD80 co-signaling molecules expressed on target cells, such as antigen-presenting PD-L2/B7-DC PD-1 ICOS cells (APCs), and their T cell-expressed receptors. Engagement of the TCR in B7-H2/ICOS L 2Ig B7-H3 (Mouse) the absence of this second co-stimulatory signal typically results in T cell B7-H1/PD-L1 B7/CD28 Families 4Ig B7-H3 (Human) anergy or apoptosis. In addition, T cell activation can be negatively regulated Unknown Receptors by co-inhibitory molecules present on APCs. Therefore, integration of the 2Ig B7-H3 Unknown B7-H4 (Mouse) Receptors signals transduced by co-stimulatory and co-inhibitory molecules following TCR B7-H5 4Ig B7-H3 engagement directs the outcome and magnitude of a T cell response Unknown Ligand (Human) B7-H5 including the enhancement or suppression of T cell proliferation, B7-H7 Unknown Receptor differentiation, and/or cytokine secretion. -

NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development
Review NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development Calum Forrest 1 , Ariane Gomes 1 , Matthew Reeves 1,* and Victoria Male 2,* 1 Institute of Immunity & Transplantation, UCL, Royal Free Campus, London NW3 2PF, UK; [email protected] (C.F.); [email protected] (A.G.) 2 Department of Metabolism, Digestion and Reproduction, Imperial College London, Chelsea and Westminster Campus, London SW10 9NH, UK * Correspondence: [email protected] (M.R.); [email protected] (V.M.) Received: 24 June 2020; Accepted: 15 July 2020; Published: 20 July 2020 Abstract: Natural killer (NK) cells are innate lymphoid cells that recognize and eliminate virally-infected and cancerous cells. Members of the innate immune system are not usually considered to mediate immune memory, but over the past decade evidence has emerged that NK cells can do this in several contexts. Of these, the best understood and most widely accepted is the response to cytomegaloviruses, with strong evidence for memory to murine cytomegalovirus (MCMV) and several lines of evidence suggesting that the same is likely to be true of human cytomegalovirus (HCMV). The importance of NK cells in the context of HCMV infection is underscored by the armory of NK immune evasion genes encoded by HCMV aimed at subverting the NK cell immune response. As such, ongoing studies that have utilized HCMV to investigate NK cell diversity and function have proven instructive. Here, we discuss our current understanding of NK cell memory to viral infection with a focus on the response to cytomegaloviruses. We will then discuss the implications that this will have for the development of a vaccine against HCMV with particular emphasis on how a strategy that can harness the innate immune system and NK cells could be crucial for the development of a vaccine against this high-priority pathogen. -

Expression Tissue
The Journal of Immunology Killer Cell Ig-Like Receptor and Leukocyte Ig-Like Receptor Transgenic Mice Exhibit Tissue- and Cell-Specific Transgene Expression1 Danny Belkin,* Michaela Torkar,* Chiwen Chang,* Roland Barten,* Mauro Tolaini,† Anja Haude,* Rachel Allen,* Michael J. Wilson,* Dimitris Kioussis,† and John Trowsdale2* To generate an experimental model for exploring the function, expression pattern, and developmental regulation of human Ig-like activating and inhibitory receptors, we have generated transgenic mice using two human genomic clones: 52N12 (a 150-Kb clone encompassing the leukocyte Ig-like receptor (LILR)B1 (ILT2), LILRB4 (ILT3), and LILRA1 (LIR6) genes) and 1060P11 (a 160-Kb clone that contains ten killer cell Ig-like receptor (KIR) genes). Both the KIR and LILR families are encoded within the leukocyte receptor complex, and are involved in immune modulation. We have also produced a novel mAb to LILRA1 to facilitate expression studies. The LILR transgenes were expressed in a similar, but not identical, pattern to that observed in humans: LILRB1 was expressed in B cells, most NK cells, and a small number of T cells; LILRB4 was expressed in a B cell subset; and LILRA1 was found on a ring of cells surrounding B cell areas on spleen sections, consistent with other data showing monocyte/macrophage expression. KIR transgenic mice showed KIR2DL2 expression on a subset of NK cells and T cells, similar to the pattern seen in humans, and expression of KIR2DL4, KIR3DS1, and KIR2DL5 by splenic NK cells. These observations indicate that linked regulatory elements within the genomic clones are sufficient to allow appropriate expression of KIRs in mice, and illustrate that the presence of the natural ligands for these receptors, in the form of human MHC class I proteins, is not necessary for the expression of the KIRs observed in these mice. -

Pan-KIR2DL NK-Receptor Antibodies and Their Use in Diagnostik and Therapy
(19) TZZ _ T (11) EP 2 287 195 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 23.02.2011 Bulletin 2011/08 C07K 16/28 (2006.01) G01N 33/52 (2006.01) (21) Application number: 10178924.6 (22) Date of filing: 01.07.2005 (84) Designated Contracting States: • Romagne, François AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 13600 La Ciotat (FR) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Wagtmann, Peter, Andreas, Nicolai, Reumert SK TR 2960 Rungsted Kyst (DK) • Svendsen, Ivan (30) Priority: 06.01.2005 DK 200500025 2765 Smorum (DK) 01.07.2004 PCT/DK2004/000470 • Zahn, Stefan 01.07.2004 PCT/IB2004/002464 2750 Ballerup (DK) • Svensson, Anders (62) Document number(s) of the earlier application(s) in 21746 Malmö (DK) accordance with Art. 76 EPC: • Thorolfsson, Matthias 05758642.2 / 1 791 868 2920 Charlottenlund (DK) • Berg Padkaer, Soren (71) Applicants: 3500 Vaerlose (DK) • Novo Nordisk A/S • Kjaergaard, Kristian 2880 Bagsvaerd (DK) 2750 Ballerup (DK) • Innate Pharma • Spee, Pieter 13009 Marseille (FR) 3450 Allerod (DK) • Universita di Genova • Wilken, Michael 16132 Genova (IT) 3390 Hundested (DK) (72) Inventors: (74) Representative: Gallois, Valérie et al • Moretta, Alessandro Cabinet BECKER & ASSOCIES 16133 Genova (IT) 25, rue Louis Le Grand • Della Chiesa, Mariella 75002 Paris (FR) 16132 Genova (IT) • Andre, Pascale Remarks: 13006 Marseille (FR) This application was filed on 23-09-2010 as a • Gauthier, Laurent divisional application to the application mentioned 13008 Marseille (FR) under INID code 62. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -
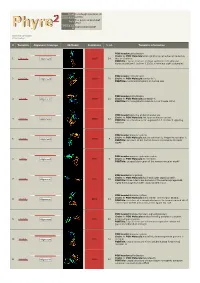
Phyre 2 Results for A1YIY0
Email [email protected] Description A1YIY0 Tue Jul 30 13:19:15 BST Date 2013 Unique Job 1035bc4b501530df ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:cell adhesion Chain: A: PDB Molecule:down syndrome cell adhesion molecule 1 c3dmkA_ Alignment 100.0 14 (dscam) isoform PDBTitle: crystal structure of down syndrome cell adhesion molecule (dscam)2 isoform 1.30.30, n-terminal eight ig domains PDB header:cell adhesion 2 c2om5A_ Alignment 100.0 20 Chain: A: PDB Molecule:contactin 2; PDBTitle: n-terminal fragment of human tax1 PDB header:cell adhesion 3 c3jxaA_ Alignment 100.0 21 Chain: A: PDB Molecule:contactin 4; PDBTitle: immunoglobulin domains 1-4 of mouse cntn4 PDB header:signaling protein/transferase Chain: A: PDB Molecule:tek tyrosine kinase variant; 4 c4k0vA_ 100.0 12 Alignment PDBTitle: structural basis for angiopoietin-1 mediated signaling initiation PDB header:immune system Chain: A: PDB Molecule:natural cytotoxicity triggering receptor 1; 5 c1p6fA_ 100.0 9 Alignment PDBTitle: structure of the human natural cytotoxicity receptor nkp46 PDB header:immune system/receptor 6 c1ollA_ Alignment 99.9 9 Chain: A: PDB Molecule:nk receptor; PDBTitle: extracellular region of the human receptor nkp46 PDB header:viral protein Chain: A: PDB Molecule:hoc head outer capsid protein; 7 c3shsA_ 99.9 18 Alignment PDBTitle: three n-terminal domains of the bacteriophage rb49 highly immunogenic2 outer capsid protein (hoc) PDB header:immune system Chain: E: PDB Molecule:natural -

Single-Cell Profiling Identifies Impaired Adaptive NK Cells Expanded After HCMV Reactivation in Haploidentical HSCT
Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical HSCT Elisa Zaghi, … , Enrico Lugli, Domenico Mavilio JCI Insight. 2021;6(12):e146973. https://doi.org/10.1172/jci.insight.146973. Research Article Hematology Immunology Graphical abstract Find the latest version: https://jci.me/146973/pdf RESEARCH ARTICLE Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical HSCT Elisa Zaghi,1 Michela Calvi,1,2 Simone Puccio,3 Gianmarco Spata,1 Sara Terzoli,1 Clelia Peano,4 Alessandra Roberto,3 Federica De Paoli,3 Jasper J.P. van Beek,3 Jacopo Mariotti,5 Chiara De Philippis,5 Barbara Sarina,5 Rossana Mineri,6 Stefania Bramanti,5 Armando Santoro,5 Vu Thuy Khanh Le-Trilling,7 Mirko Trilling,7 Emanuela Marcenaro,8 Luca Castagna,5 Clara Di Vito,1,2 Enrico Lugli,3,9 and Domenico Mavilio1,2 1Unit of Clinical and Experimental Immunology, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy. 2BIOMETRA, Università degli Studi di Milano, Milan, Italy. 3Laboratory of Translational Immunology, 4Institute of Genetic and Biomedical Research, UoS Milan, National Research Council, and Genomic Unit, 5Bone Marrow Transplant Unit, and 6Molecular Biology Section, Clinical Investigation Laboratory, IRCCS Humanitas Research Hospital, Milan, Italy. 7Institute for Virology, University Hospital Essen, University Duisburg-Essen, Essen, Germany. 8Department of Experimental Medicine, University of Genoa, Genoa, Italy. 9Flow Cytometry Core, IRCCS Humanitas Research Hospital, Milan, Italy. Haploidentical hematopoietic stem cell transplantation (h-HSCT) represents an efficient curative approach for patients affected by hematologic malignancies in which the reduced intensity conditioning induces a state of immunologic tolerance between donor and recipient. -

Natural Killer Cells in Antiviral Immunity
REVIEWS Natural killer cells in antiviral immunity Niklas K. Björkström ✉ , Benedikt Strunz and Hans- Gustaf Ljunggren Abstract | Natural killer (NK) cells play an important role in innate immune responses to viral infections. Here, we review recent insights into the role of NK cells in viral infections, with particular emphasis on human studies. We first discuss NK cells in the context of acute viral infections, with flavivirus and influenza virus infections as examples. Questions related to activation of NK cells, homing to infected tissues and the role of tissue- resident NK cells in acute viral infections are also addressed. Next, we discuss NK cells in the context of chronic viral infections with hepatitis C virus and HIV-1. Also covered is the role of adaptive- like NK cell expansions as well as the appearance of CD56− NK cells in the course of chronic infection. Specific emphasis is then placed in viral infections in patients with primary immunodeficiencies affecting NK cells. Not least, studies in this area have revealed an important role for NK cells in controlling several herpesvirus infections. Finally, we address new data with respect to the activation of NK cells and NK cell function in humans infected with severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) giving rise to coronavirus disease 2019 (COVID-19). Antibody- dependent Almost 50 years ago, a small number of research groups In humans, mature NK cells are traditionally identified cellular cytotoxicity started to observe unexpected spontaneous cytotoxic as CD3−CD56+ lymphocytes. They were long thought to (ADCC). A mechanism by which activities among lymphocytes. -

Genitourinary Pathology (Including Renal Tumors)
LABORATORY INVESTIGATION THE BASIC AND TRANSLATIONAL PATHOLOGY RESEARCH JOURNAL LI VOLUME 99 | SUPPLEMENT 1 | MARCH 2019 2019 ABSTRACTS GENITOURINARY PATHOLOGY (INCLUDING RENAL TUMORS) (776-992) MARCH 16-21, 2019 PLATF OR M & 2 01 9 ABSTRACTS P OSTER PRESENTATI ONS EDUCATI ON C O M MITTEE Jason L. Hornick , C h air Ja mes R. Cook R h o n d a K. Y a nti s s, Chair, Abstract Revie w Board S ar a h M. Dr y and Assign ment Co m mittee Willi a m C. F a q ui n Laura W. La mps , Chair, C ME Subco m mittee C ar ol F. F ar v er St e v e n D. Billi n g s , Interactive Microscopy Subco m mittee Y uri F e d ori w Shree G. Shar ma , Infor matics Subco m mittee Meera R. Ha meed R aj a R. S e et h al a , Short Course Coordinator Mi c h ell e S. Hir s c h Il a n W ei nr e b , Subco m mittee for Unique Live Course Offerings Laksh mi Priya Kunju D a vi d B. K a mi n s k y ( Ex- Of ici o) A n n a M ari e M ulli g a n Aleodor ( Doru) Andea Ri s h P ai Zubair Baloch Vi nita Parkas h Olca Bast urk A nil P ar w a ni Gregory R. Bean , Pat h ol o gist-i n- Trai ni n g D e e p a P atil D a ni el J. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like,