Supporting Info
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Single-Cell Profiling Identifies Impaired Adaptive NK Cells Expanded After HCMV Reactivation in Haploidentical HSCT
Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical HSCT Elisa Zaghi, … , Enrico Lugli, Domenico Mavilio JCI Insight. 2021;6(12):e146973. https://doi.org/10.1172/jci.insight.146973. Research Article Hematology Immunology Graphical abstract Find the latest version: https://jci.me/146973/pdf RESEARCH ARTICLE Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical HSCT Elisa Zaghi,1 Michela Calvi,1,2 Simone Puccio,3 Gianmarco Spata,1 Sara Terzoli,1 Clelia Peano,4 Alessandra Roberto,3 Federica De Paoli,3 Jasper J.P. van Beek,3 Jacopo Mariotti,5 Chiara De Philippis,5 Barbara Sarina,5 Rossana Mineri,6 Stefania Bramanti,5 Armando Santoro,5 Vu Thuy Khanh Le-Trilling,7 Mirko Trilling,7 Emanuela Marcenaro,8 Luca Castagna,5 Clara Di Vito,1,2 Enrico Lugli,3,9 and Domenico Mavilio1,2 1Unit of Clinical and Experimental Immunology, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy. 2BIOMETRA, Università degli Studi di Milano, Milan, Italy. 3Laboratory of Translational Immunology, 4Institute of Genetic and Biomedical Research, UoS Milan, National Research Council, and Genomic Unit, 5Bone Marrow Transplant Unit, and 6Molecular Biology Section, Clinical Investigation Laboratory, IRCCS Humanitas Research Hospital, Milan, Italy. 7Institute for Virology, University Hospital Essen, University Duisburg-Essen, Essen, Germany. 8Department of Experimental Medicine, University of Genoa, Genoa, Italy. 9Flow Cytometry Core, IRCCS Humanitas Research Hospital, Milan, Italy. Haploidentical hematopoietic stem cell transplantation (h-HSCT) represents an efficient curative approach for patients affected by hematologic malignancies in which the reduced intensity conditioning induces a state of immunologic tolerance between donor and recipient. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

High-Dimensional Analysis Reveals a Distinct Population of Adaptive-Like Tissue
bioRxiv preprint doi: https://doi.org/10.1101/2019.12.20.883785; this version posted December 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 High-dimensional analysis reveals a distinct population of adaptive-like tissue- 2 resident NK cells in human lung 3 4 Nicole Marquardt1*, Marlena Scharenberg1, Jeffrey E. Mold2, Joanna Hård2, Eliisa 5 Kekäläinen3,4, Marcus Buggert1, Son Nguyen5,6, Jennifer N. Wilson1, Mamdoh Al- 6 Ameri7, Hans-Gustaf Ljunggren1, Jakob Michaëlsson1 7 8 Running title: Adaptive-like human lung tissue-resident NK cells 9 10 Affiliations: 11 1Center for Infectious Medicine, Department of Medicine, Karolinska Institutet, 12 Stockholm, Sweden 13 2Department of Cell and Molecular Biology, Karolinska Institutet, Stockholm, Sweden 14 3Translational Immunology Research Program & Department of Bacteriology and 15 Immunology, University of Helsinki, Finland 16 4HUSLAB, Division of Clinical Microbiology, Helsinki University Hospital, Helsinki, 17 Finland, 18 5Department of Microbiology, Perelman School of Medicine, University of 19 Pennsylvania, Philadelphia, PA, USA 20 6Institute for Immunology, Perelman School of Medicine, University of Pennsylvania, 21 Philadelphia, PA, USA 22 7Thoracic Surgery, Department of Molecular Medicine and Surgery, Karolinska 23 Institutet, Karolinska University Hospital, Stockholm, Sweden 24 1 bioRxiv preprint doi: https://doi.org/10.1101/2019.12.20.883785; this version posted December 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Type of the Paper (Article
Supplementary figures and tables E g r 1 F g f2 F g f7 1 0 * 5 1 0 * * e e e * g g g * n n n * a a a 8 4 * 8 h h h * c c c d d d * l l l o o o * f f f * n n n o o o 6 3 6 i i i s s s s s s e e e r r r p p p x x x e e e 4 2 4 e e e n n n e e e g g g e e e v v v i i i t t t 2 1 2 a a a l l l e e e R R R 0 0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d J a k 2 N o tc h 2 H if1 * 3 4 6 * * * e e e g g g n n n a a * * a * h h * h c c c 3 * d d * d l l l * o o o f f 2 f 4 n n n o o o i i i s s s s s s e e e r r 2 r p p p x x x e e e e e e n n n e e 1 e 2 g g g e e 1 e v v v i i i t t t a a a l l l e e e R R R 0 0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d Z e b 2 C d h 1 S n a i1 * * 7 1 .5 4 * * e e e g g g 6 n n n * a a a * h h h c c c 3 * d d d l l l 5 o o o f f f 1 .0 * n n n * o o o i i i 4 * s s s s s s e e e r r r 2 p p p x x x 3 e e e e e e n n n e e e 0 .5 g g g 2 e e e 1 v v v i i i t t t a a a * l l l e e e 1 * R R R 0 0 .0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d M m p 9 L o x V im 2 0 0 2 0 8 * * * e e e * g g g 1 5 0 * n n n * a a a * h h h * c c c 1 5 * 6 d d d l l l 1 0 0 o o o f f f n n n o o o i i i 5 0 s s s s s s * e e e r r r 1 0 4 3 0 p p p * x x x e e e * e e e n n n e e e 2 0 g g g e e e 5 2 v v v i i i t t t a a a l l l 1 0 e e e R R R 0 0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d Supplementary Figure 1. -

Potential for Natural Killer Cell-Mediated Antibody-Dependent Cellular Cytotoxicity for Control of Human Cytomegalovirus
Antibodies 2013, 2, 617-635; doi:10.3390/antib2040617 OPEN ACCESS antibodies ISSN 2073-4468 www.mdpi.com/journal/antibodies Review Potential for Natural Killer Cell-Mediated Antibody-Dependent Cellular Cytotoxicity for Control of Human Cytomegalovirus Rebecca J. Aicheler *, Eddie C. Y. Wang, Peter Tomasec, Gavin W. G. Wilkinson and Richard J. Stanton Cardiff Institute of Infection and Immunity, Cardiff University, Cardiff, CF14 4XN, UK; E-Mails: [email protected] (E.C.Y.W.); [email protected] (P.T.); [email protected] (G.W.G.W.); [email protected] (R.J.S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +44-0-292-068-7319. Received: 31 October 2013; in revised form: 15 November 2013 / Accepted: 27 November 2013 / Published: 10 December 2013 Abstract: Human cytomegalovirus (HCMV) is an important pathogen that infects the majority of the population worldwide, yet, currently, there is no licensed vaccine. Despite HCMV encoding at least seven Natural Killer (NK) cell evasion genes, NK cells remain critical for the control of infection in vivo. Classically Antibody-Dependent Cellular Cytotoxicity (ADCC) is mediated by CD16, which is found on the surface of the NK cell in a complex with FcεRI-γ chains and/or CD3ζ chains. Ninety percent of NK cells express the Fc receptor CD16; thus, they have the potential to initiate ADCC. HCMV has a profound effect on the NK cell repertoire, such that up to 10-fold expansions of NKG2C+ cells can be seen in HCMV seropositive individuals. These NKG2C+ cells are reported to be FcεRI-γ deficient and possess variable levels of CD16+, yet have striking ADCC functions. -

Expansions of Adaptive-Like NK Cells with a Tissue-Resident Phenotype in Human Lung and Blood
Expansions of adaptive-like NK cells with a tissue-resident phenotype in human lung and blood Demi Brownliea,1, Marlena Scharenberga,1, Jeff E. Moldb, Joanna Hårdb, Eliisa Kekäläinenc,d,e, Marcus Buggerta, Son Nguyenf,g, Jennifer N. Wilsona, Mamdoh Al-Amerih, Hans-Gustaf Ljunggrena, Nicole Marquardta,2,3, and Jakob Michaëlssona,2 aCenter for Infectious Medicine, Department of Medicine Huddinge, Karolinska Institutet, 14152 Stockholm, Sweden; bDepartment of Cell and Molecular Biology, Karolinska Institutet, 171 77 Stockholm, Sweden; cTranslational Immunology Research Program, University of Helsinki, 00014 Helsinki, Finland; dDepartment of Bacteriology and Immunology, University of Helsinki, 00014 Helsinki, Finland; eHelsinki University Central Hospital Laboratory, Division of Clinical Microbiology, Helsinki University Hospital, 00290 Helsinki, Finland; fDepartment of Microbiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104; gInstitute for Immunology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104; and hThoracic Surgery, Department of Molecular Medicine and Surgery, Karolinska University Hospital, Karolinska Institutet, 171 76 Stockholm, Sweden Edited by Marco Colonna, Washington University in St. Louis School of Medicine, St. Louis, MO, and approved January 27, 2021 (received for review August 18, 2020) Human adaptive-like “memory” CD56dimCD16+ natural killer (NK) We and others recently identified a subset of tissue-resident − cells in peripheral blood from cytomegalovirus-seropositive indi- CD49a+CD56brightCD16 NK cells in the human lung (14, 15). viduals have been extensively investigated in recent years and are The human lung is a frequent site of infection with viruses such currently explored as a treatment strategy for hematological can- as influenza virus and HCMV, as well as a reservoir for latent cers. -

Differences in Gene Expression Profiles and Carcinogenesis Pathways Involved in Cisplatin Resistance of Four Types of Cancer
596 ONCOLOGY REPORTS 30: 596-614, 2013 Differences in gene expression profiles and carcinogenesis pathways involved in cisplatin resistance of four types of cancer YONG YANG1,2, HUI LI1,2, SHENGCAI HOU1,2, BIN HU1,2, JIE LIU1,3 and JUN WANG1,3 1Beijing Key Laboratory of Respiratory and Pulmonary Circulation, Capital Medical University, Beijing 100069; 2Department of Thoracic Surgery, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100020; 3Department of Physiology, Capital Medical University, Beijing 100069, P.R. China Received December 23, 2012; Accepted March 4, 2013 DOI: 10.3892/or.2013.2514 Abstract. Cisplatin-based chemotherapy is the standard Introduction therapy used for the treatment of several types of cancer. However, its efficacy is largely limited by the acquired drug Cisplatin is primarily effective through DNA damage and is resistance. To date, little is known about the RNA expression widely used for the treatment of several types of cancer, such changes in cisplatin-resistant cancers. Identification of the as testicular, lung and ovarian cancer. However, the ability RNAs related to cisplatin resistance may provide specific of cancer cells to become resistant to cisplatin remains a insight into cancer therapy. In the present study, expression significant impediment to successful chemotherapy. Although profiling of 7 cancer cell lines was performed using oligo- previous studies have identified numerous mechanisms in nucleotide microarray analysis data obtained from the GEO cisplatin resistance, it remains a major problem that severely database. Bioinformatic analyses such as the Gene Ontology limits the usefulness of this chemotherapeutic agent. Therefore, (GO) and KEGG pathway were used to identify genes and it is crucial to examine more elaborate mechanisms of cisplatin pathways specifically associated with cisplatin resistance. -

Effect of NKG2C Absence on Natural Killer Cell Phenotype and Function in Human
Effect of NKG2C Absence on Natural Killer Cell Phenotype and Function in Human Immunodeficiency Virus/Cytomegalovirus Co-infection ©Emilie M. Comeau A thesis submitted to the School of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science in Medicine Division of BioMedical Science Immunology and Infectious Diseases Faculty of Medicine, Memorial University of Newfoundland St. John’s, Newfoundland May 2018 Abstract Natural killer (NK) cells expressing NKG2C and CD57 expand following human cytomegalovirus (CMV) infection. This NK cell subset downregulates FcεR1γ and acquires enhanced capacity to mediate antibody-dependent cellular cytotoxicity (ADCC). Expansion of these differentiated NK cells is exaggerated in human immunodeficiency virus (HIV) infection. Individuals lacking the gene encoding NKG2C have diminished resistance to HIV, but it remains unclear whether differentiation into NK cells with superior ADCC is impaired in NKG2Cnull individuals. Therefore, our objective was to investigate if CMV-driven NK cell differentiation into enhanced killers is impaired in NKG2Cnull HIV-infected individuals. Phenotypic (CD57+, FcεR1γ-) and functional (IFN- γ, TNF-α induction and cytotoxicity) NK cell responses were compared between NKG2Cnull and matched NKG2C-expressing individuals by flow cytometry following stimulation through natural cytotoxicity receptors (using K562 cells) or CD16 (using monoclonal antibody, 3G8). Cytotoxicity was measured in 51Chromium release assays against anti-CD16-coated P815 cells (redirected lysis) and anti-human leukocyte antigen (HLA) class I antibody-coated C1R cells (classical ADCC). Antibodies were titrated to determine concentrations producing half maximal responses (EC50) to compare sensitivity. Our data indicate highly similar CMV-driven NK cell differentiation in terms of both general phenotype and function, regardless of NKG2C genotype. -

Mouse CD Marker Chart Bdbiosciences.Com/Cdmarkers
BD Mouse CD Marker Chart bdbiosciences.com/cdmarkers 23-12400-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1d CD1.1, CD1.2, Ly-38 Lipid, Glycolipid Ag + + + + + + + + CD104 Integrin b4 Laminin, Plectin + DNAX accessory molecule 1 (DNAM-1), Platelet and T cell CD226 activation antigen 1 (PTA-1), T lineage-specific activation antigen 1 CD112, CD155, LFA-1 + + + + + – + – – CD2 LFA-2, Ly-37, Ly37 CD48, CD58, CD59, CD15 + + + + + CD105 Endoglin TGF-b + + antigen (TLiSA1) Mucin 1 (MUC1, MUC-1), DF3 antigen, H23 antigen, PUM, PEM, CD227 CD54, CD169, Selectins; Grb2, β-Catenin, GSK-3β CD3g CD3g, CD3 g chain, T3g TCR complex + CD106 VCAM-1 VLA-4 + + EMA, Tumor-associated mucin, Episialin + + + + + + Melanotransferrin (MT, MTF1), p97 Melanoma antigen CD3d CD3d, CD3 d chain, T3d TCR complex + CD107a LAMP-1 Collagen, Laminin, Fibronectin + + + CD228 Iron, Plasminogen, pro-UPA (p97, MAP97), Mfi2, gp95 + + CD3e CD3e, CD3 e chain, CD3, T3e TCR complex + + CD107b LAMP-2, LGP-96, LAMP-B + + Lymphocyte antigen 9 (Ly9), -
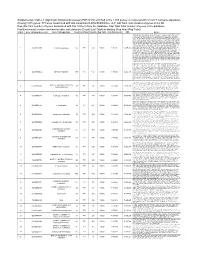
(FDR<0.05) Enriched in the 1015 Genes Co-Clustered with Known T
Supplementary Table 3. Significant biological processes (FDR<0.05) enriched in the 1,015 genes co-clustered with known T cell gene signatures. Among 1,015 genes, 771 were associated with GO annotation in DAVID database v6.7. List Total: total number of genes in my list. Pop Hits: total number of genes associated with this GO term from the database. Pop Total: total number of genes in the database. Fold Enrichment: relative enrichment ratio, calculated by (Count)/(List Total) divided by (Pop Hits)/(Pop Total). Index Gene Ontology Accession Gene Ontology Name Count List Total Pop Hits Pop Total Fold Enrichment FDR Genes AQP9, C1QC, B2M, LILRA1, LILRA2, CLEC4E, S1PR4, LILRA4, IFNG, LILRA6, CLEC4A, VNN1, ERAP2, FAS, CRTAM, C5AR1, GBP5, NCF2, NCF1, NCF4, SERPING1, HLA-DQA2, HLA-DQA1, PDCD1LG2, LILRB1, CCR9, C1QA, C1QB, LILRB2, CCR7, CCR6, UNC13D, CCR5, CD40LG, CCR4, LILRB3, CCR2, LILRB4, HLA-DPA1, VSIG4, HLA-DRA, IL1R2, IL1R1, HLA-DRB1, OAS3, ACP5, OAS1, OAS2, CD74, IFI35, ZAP70, FCER1G, HLA-DRB5, HLA-DPB1, HLA-DOA, HLA-DOB, DHX58, BLNK, IL23R, KIR2DS4, CD300C, SLAMF7, OASL, RGS1, APOL1, CD300A, HMHB1, CD209, CLEC7A, LY86, LY9, CLNK, FCRL4, SH2D1A, NOD2, HAMP, CCL3L1, CCL3L3, TICAM2, ICAM1, GZMA, CMKLR1, LY96, WAS, IL18BP, LAX1, TNFSF12- TNFSF13, HLA-DQB1, CSF2, GPR183, CCR1, GPR65, CXCL9, NCF1C, IL7R, CLEC10A, CCL24, CCL22, CYP27B1, CCL23, FCGR1C, FTHL3, FCGR1A, FCGR1B, BCL3, C2, CD27, CD28, FYB, IL18R1, IL7, CD1C, CTLA4, CCL19, CD1B, CD1A, TRIM22, CD180, CD1E, CCL18, CCL17, CCL13, FCGR2B, FCGR2C, P2RY14, LIME1, CD14, IL16, IL18, TLR1, TNFSF15, -

Table S4. RAE Analysis of Dedifferentiated Liposarcoma
Table S4. RAE analysis of dedifferentiated liposarcoma Model Chromosome Region start Region end Size q value freqX0* # genes genes Amp 1 57809872 60413476 2603605 0.00026 34.6 10 DAB1,RPS26P15,OMA1,TACSTD2,MYSM1,JUN,FGGY,HOOK1,CYP2J2,C1orf87 Amp 1 158619146 158696968 77823 0.053 25 1 VANGL2 Amp 1 158883523 158922841 39319 0.081 23.1 2 SLAMF1,CD48 Amp 1 162042586 162118557 75972 0.072 25 0 [Nearest:NUF2] Amp 1 162272460 162767627 495168 0.017 26.9 0 [Nearest:PBX1] Amp 1 165486554 165532374 45821 0.057 25 1 POU2F1 Amp 1 167138282 167483267 344986 0.024 26.9 2 ATP1B1,NME7 Amp 1 167612872 167708844 95973 0.041 25 3 BLZF1,C1orf114,SLC19A2 Amp 1 167728199 167808161 79963 0.076 21.2 1 F5 Amp 1 168436370 169233893 797524 0.018 26.9 3 GORAB,PRRX1,C1orf129 Amp 1 169462231 170768440 1306210 1.3E-06 38.5 10 FMO1,FMO4,TOP1P1,BAT2D1,MYOC,VAMP4,METTL13,DNM3,C1orf105,PIGC Amp 1 171026247 171291427 265181 0.015 26.9 1 TNFSF18 Del 1 201860394 202299299 438906 0.0047 25 6 ATP2B4,SNORA77,LAX1,ZC3H11A,SNRPE,C1orf157 Del 1 210909187 212021116 1111930 0.017 19.2 8 BATF3,NSL1,TATDN3,C1orf227,FLVCR1,VASH2,ANGEL2,RPS6KC1 Del 1 215937857 216049214 111358 0.079 23.1 1 SPATA17 Del 1 218237257 218367476 130220 0.0063 26.9 3 EPRS,BPNT1,IARS2 Del 1 222100886 222727238 626353 5.2E-05 32.7 5 FBXO28,DEGS1,NVL,CNIH4,WDR26 Del 1 223166548 224519805 1353258 0.0063 26.9 15 DNAH14,LBR,ENAH,SRP9,EPHX1,TMEM63A,LEFTY1,PYCR2,LEFTY2,C1orf55,H3F3A,LOC440926 ,ACBD3,MIXL1,LIN9 Del 1 225283136 225374166 91031 0.054 23.1 1 CDC42BPA Del 1 227278990 229012661 1733672 0.091 21.2 13 RAB4A,SPHAR,C1orf96,ACTA1,NUP133,ABCB10,TAF5L,URB2,GALNT2,PGBD5,COG2,AGT,CAP -

Human Gene Expression Profiles of Susceptibility and Resistance in Tuberculosis
Genes and Immunity (2011) 12, 15–22 & 2011 Macmillan Publishers Limited All rights reserved 1466-4879/11 www.nature.com/gene ORIGINAL ARTICLE Human gene expression profiles of susceptibility and resistance in tuberculosis J Maertzdorf1, D Repsilber2, SK Parida1, K Stanley3, T Roberts3, G Black3, G Walzl3 and SHE Kaufmann1 1Department of Immunology, Max Planck Institute for Infection Biology, Berlin, Germany; 2Leibniz Institute for Farm Animal Biology, Genetics and Biometry, Dummerstorf, Germany and 3Division of Molecular Biology and Human Genetics, MRC Centre for Molecular and Cellular Biology, DST and NRF Centre of Excellence for Biomedical TB Research, Faculty of Health Sciences, Stellenbosch University, Cape Town, South Africa Tuberculosis (TB) still poses a profound burden on global health, owing to significant morbidity and mortality worldwide. Although a fully functional immune system is essential for the control of Mycobacterium tuberculosis infection, the underlying mechanisms and reasons for failure in part of the infected population remain enigmatic. Here, whole-blood microarray gene expression analyses were performed in TB patients and in latently as well as uninfected healthy controls to define biomarkers predictive of susceptibility and resistance. Fc gamma receptor 1B (FCGRIB)was identified as the most differentially expressed gene, and, in combination with four other markers, produced a high degree of accuracy in discriminating TB patients and latently infected donors. We determined differentially expressed genes unique for active disease and identified profiles that correlated with susceptibility and resistance to TB. Elevated expression of innate immune-related genes in active TB and higher expression of particular gene clusters involved in apoptosis and natural killer cell activity in latently infected donors are likely to be the major distinctive factors determining failure or success in controlling M.