Vapor Depostion Process for Depositing an Organo-Metallic Compound Layer on a Substrate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gasb Paper R2 ACS Nano
High-Mobility GaSb Nanostructures Cointegrated with InAs on Si Borg, Mattias; Schmid, Heinz; Gooth, Johannes; Rossell, Marta D.; Cutaia, Davide; Knoedler, Moritz; Bologna, Nicolas; Wirths, Stephan; Moselund, Kirsten E.; Riel, Heike Published in: ACS Nano DOI: 10.1021/acsnano.6b04541 2017 Document Version: Peer reviewed version (aka post-print) Link to publication Citation for published version (APA): Borg, M., Schmid, H., Gooth, J., Rossell, M. D., Cutaia, D., Knoedler, M., Bologna, N., Wirths, S., Moselund, K. E., & Riel, H. (2017). High-Mobility GaSb Nanostructures Cointegrated with InAs on Si. ACS Nano, 11(3), 2554- 2560. https://doi.org/10.1021/acsnano.6b04541 Total number of authors: 10 Creative Commons License: Unspecified General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Gallium Antimonide-Doped Germanium Clathrate-A P-Type
Journal of Solid State Chemistry 151, 61}64 (2000) doi:10.1006/jssc.2000.8622, available online at http://www.idealibrary.com on Gallium Antimonide-Doped Germanium Clathrated A p-Type Thermoelectric Cage Structure Susan Latturner, Xianhui Bu, Nick Blake, Horia Metiu, and Galen Stucky Department of Chemistry, University of California, Santa Barbara, California 93106 Received October 5, 1999; in revised form December 27, 1999; accepted January 10, 2000 BaGaGe in which the group 13 metal acts as an elec- A germanium clathrate compound doped with gallium anti- tron-accepting site (6,7). In the apparently charge-unbal- monide has been synthesized and characterized. The structure of anced structures such as K Sn , vacancies have been found ؍ Ba8Ga16(GaSb)xGe30؊2x with x 2 was determined using single- on speci"c framework sites, leading to an actual crystal X-ray analysis, and the Sb atoms were found to be stoichiometry of KSn[], where the three-bonded tin selectively sited. A slight excess of antimony was con5rmed by atoms act as electron-accepting sites (8). Despite this evid- microprobe elemental analysis, resulting in uncompensated dop- ence, the hypothesis of full charge transfer from guest atom ing of the framework. A positive and relatively high Seebeck to host framework to form a closed-shell zintl phase has coe7cient of 100 lV/K indicates this compound is a potential p-type thermoelectric material. Single-crystal temperature-de- been called into question by recent experiments which indi- pendent conductivity indicates that the sample behaves as a poor cate that the guest is close to neutral in some cases (2, 9). -

Gallium in Semiconductors When the Word 'Semiconductor' Is Mentioned
Gallium in Semiconductors When the word ‘semiconductor’ is mentioned, it is normal to think of silicon, as it is the key material used to make transistors, integrated circuits, and other related components. While silicon is indeed the most common semiconductor in electronic device application , it is not alone, and other elements and compounds have also found use. In fact, the first transistor was made from germanium and throughout the 1950s it was the main material in transistors and other semiconductor devices. However, silicon was also known to be suitable for producing semiconductors and, once its processing was optimised, it rapidly replaced germanium (although it does still find use in some specialised applications). Electronic devices do not have to be made from a single element; they can be fabricated from what are known as compound semiconductors. Instead of using a group four element such as silicon or germanium, a compound made by combining a group three and a group five element can be utilised. These so-called ‘III-V’ compounds have been developed for use in electronics since the 1970s, with the most popular one being gallium arsenide (GaAs) (see Figure 1). Figure 1 The zinc blend crystal structure of gallium arsenide GaAs offers several advantages over silicon, making it more suitable for some applications and especially those requiring high frequency operation. GaAs components function up to around 250 GHz, meaning that they can support the signal speeds needed for the successful operation of 5G networks. They are also not affected as much by high temperatures as silicon, and they generate less noise than silicon when operating at elevated frequencies. -

APPLIED PHYSICS REVIEWS the Physics and Technology of Gallium
APPLIED PHYSICS REVIEWS The physics and technology of gallium antimonide: An emerging optoelectronic material P. S. Duttaa) and H. L. Bhat Department of Physics, Indian Institute of Science, Bangalore-560 012, India Vikram Kumar Solid State Physics Laboratory, Lucknow Road, Delhi-110 054, India ~Received 22 February 1996; accepted for publication 20 January 1997! Recent advances in nonsilica fiber technology have prompted the development of suitable materials for devices operating beyond 1.55 mm. The III–V ternaries and quaternaries ~AlGaIn!~AsSb! lattice matched to GaSb seem to be the obvious choice and have turned out to be promising candidates for high speed electronic and long wavelength photonic devices. Consequently, there has been tremendous upthrust in research activities of GaSb-based systems. As a matter of fact, this compound has proved to be an interesting material for both basic and applied research. At present, GaSb technology is in its infancy and considerable research has to be carried out before it can be employed for large scale device fabrication. This article presents an up to date comprehensive account of research carried out hitherto. It explores in detail the material aspects of GaSb starting from crystal growth in bulk and epitaxial form, post growth material processing to device feasibility. An overview of the lattice, electronic, transport, optical and device related properties is presented. Some of the current areas of research and development have been critically reviewed and their significance for both understanding the basic physics as well as for device applications are addressed. These include the role of defects and impurities on the structural, optical and electrical properties of the material, various techniques employed for surface and bulk defect passivation and their effect on the device characteristics, development of novel device structures, etc. -

Gallium Antimonide (Gasb)
Gallium antimonide (GaSb) Volodymyr Nahirnyak Department of Physics University of Cincinnati Cincinnati, Ohio 45221 March 17, 2002 Abstract Although GaSb has not been widely utilized in technological applications, its energy band structure does allow various interesting physical experiments to be per- formed on intervalley transfer mechanisms and thus the nature of L and X energetic minima in the Ga series of III-V semiconductor compounds can be clari¯ed. 1 1 Crystalline structure The crystal lattice of CaSb belongs to the zinc blende lattice and is constituted by two fcc 3 sublattices formed by atoms Ga and Sb. The density of the crystal is 5.61 g cm¡ and ¢ the lattice constant ao = 6.09593 A_ [1] and the melting point T = 985 K [2] and Debye temperature D - 266K. The zinc blende structure of GaSb is based on the diamond lattice but the two di®erent atoms in primitive cell.The two fcc sublattices can be de¯ned by basic translational vec- tors: ao/2(1,1,0), ao/2(0,1,1), ao/2(1,0,1) and are displaced by vector d=ao/4(1,1,1) with respect to one another. The bonding forces in such semiconductor compounds are partially covalent and partially ionic. Although the zinc blende structure is closely related to the diamond structure , there is an important di®erence between the two: the diamond lattice has a center of inversion midway between two atoms in primitive cell, whereas the zinc blende lattice does not. This has implications for the nonlinear optical properties of GaSb: only crystals lacking a center of inversion can be strongly nonlinear[4]. -

Direct Band Gap Gallium Antimony Phosphide (Gasbxp1-X) Alloys
University of Kentucky UKnowledge Center for Computational Sciences Faculty Center for Computational Sciences Publications 2-10-2016 Direct Band Gap Gallium Antimony Phosphide (GaSbxP1-x) Alloys H. B. Russell University of Louisville A. N. Andriotis Foundation for Research & Technology - Hellas (FORTH), Greece Madhu Menon University of Kentucky, [email protected] J. B. Jasinski University of Louisville A. Martinez-Garcia University of Louisville See next page for additional authors Click here to let us know how access to this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/ccs_facpub Part of the Chemical Engineering Commons, Computer Sciences Commons, and the Physics Commons Repository Citation Russell, H. B.; Andriotis, A. N.; Menon, Madhu; Jasinski, J. B.; Martinez-Garcia, A.; and Sunkara, M. K., "Direct Band Gap Gallium Antimony Phosphide (GaSbxP1-x) Alloys" (2016). Center for Computational Sciences Faculty Publications. 5. https://uknowledge.uky.edu/ccs_facpub/5 This Article is brought to you for free and open access by the Center for Computational Sciences at UKnowledge. It has been accepted for inclusion in Center for Computational Sciences Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Authors H. B. Russell, A. N. Andriotis, Madhu Menon, J. B. Jasinski, A. Martinez-Garcia, and M. K. Sunkara Direct Band Gap Gallium Antimony Phosphide (GaSbxP1-x) Alloys Notes/Citation Information Published in Scientific Reports, v. 6, article no. 20822, p. 1-9. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. -

High Purity Tri-Methyl Gallium (99.999%)
HIGH PURITY TRI-METHYL GALLIUM (99.999%) Trimethylgallium, Ga(CH3)3 , often abbreviated to TMG or TMGa, commonly used metalorganic source of gallium for metalorganic vapour phase epitaxy (MOVPE) for preparation of gallium-containing compound semiconductors used in fabricaton of optoelectronic devices and solar panels. In our synthethetic method the intermediate adduct of TMG is prepared which has no such limitation & can be stored for long time without any stringent safety precautions. The emphasis of Government on semiconductor chips manufacturing in India, will boost the demand indigenously sourced raw materials. Specifications: • TMG is a clear, colorless, pyrophoric liquid ie. catch fire on exposure to air. TMG is known to react violently with water and other compounds that are capable of providing labile and active hydrogen (i.e. protons). • TMG needs to be handled with care and caution, e.g. stored in a cool, dry place at 0-25 °C, under inert atmosphere, and ensuring that storage temperatures would not exceed 40 °C to avoid deterioration. Application: • Trimethylgallium, Ga(CH3)3, TMG or TMGa is commonly used metalorganic source of gallium for metalorganic vapour phase epitaxy (MOVPE) for preparation of gallium-containing compound semiconductors, such as:- • gallium arsenide (GaAs) (used in space applications, blue LED material galium nitride (GaN), • gallium phosphide (GaP), gallium antimonide (GaSb) (used in fabrication of optoelectronic devices), • copper indium gallium sulfide and copper indium gallium selenide (CIGS) (used in manufacture of high efficiency solar panels). • TMG is used for preparation of thin films of gallium based semiconductor materials which are used in micro-electronic and high speed devices. -

Scanning Tunneling Microscopy of Metals and Semiconductors
Scanning Tunneling Microscopy of Metals and Semiconductors Introduction Applications of scanning tunneling microscopy (STM) to surfaces of both metals and semiconductors have rapidly expanded during the last fifteen years. Initial STM studies in both cases focused on structural imaging as well as measurement of electronic properties using scanning tunneling spectroscopy (STS). That early work was primarily for low-index surfaces, as reviewed by Hamers [97H1]. Later studies included a much greater range of surfaces and measurement techniques. The following literature overview summarizes the STM/STS studies of crystalline surfaces of metals and semiconductors from 1997 to 2012. The elements and compounds are listed with alphabetical order, and within each material the topics are ordered from lower to higher index surfaces. Metals The metal surfaces are rich in physical phenomena and many metallic surfaces are important for adsorbate ordering, nanostructure formation and surface catalysis processes. The focus of STM studies on metal surfaces from 1997 to 2012 varies depending on the type of metal surface or surface index. On some metal surfaces such as Ag(111) and Cu(111), the existence of a two dimensional surface state near the Fermi level influences adatom diffusion, epitaxial growth, equilibrium crystal shapes, reconstructions, surface chemical reactions, and adsorbate ordering processes. STM with its imaging and spectroscopic capabilities is the right choice of instrument to explore the surface state related phenomena on these surfaces, including the surface state lifetime, surface state electron confinement, and the electron standing waves. STM is also used to study the image-potential states on metal surfaces. The ‘z’ versus V spectroscopic data show the existence of Rydberg-like series of potentials along the surface normal direction on many metal surfaces. -

Electrical Characterization of Deuterium-Doped Gallium Antimonide Grown on Silicon Substrates Justin T
University of Vermont ScholarWorks @ UVM UVM Honors College Senior Theses Undergraduate Theses 2014 Electrical Characterization of Deuterium-Doped Gallium Antimonide Grown on Silicon Substrates Justin T. Dao University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/hcoltheses Recommended Citation Dao, Justin T., "Electrical Characterization of Deuterium-Doped Gallium Antimonide Grown on Silicon Substrates" (2014). UVM Honors College Senior Theses. 5. https://scholarworks.uvm.edu/hcoltheses/5 This Honors College Thesis is brought to you for free and open access by the Undergraduate Theses at ScholarWorks @ UVM. It has been accepted for inclusion in UVM Honors College Senior Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. Electrical Characterization of Deuterium-Doped Gallium Antimonide Grown on Silicon Substrates Justin Dao Honors Thesis Electrical Engineering University of Vermont Advisor: Professor Walter Varhue, Electrical Engineering 1 Abstract In the world today, there exists the need for advances in high speed, energy efficient electronic applications. In the fields of lasers, transistors, and thermophotovoltaic systems, one potential improvement that is currently being explored is the use of gallium antimonide (GaSb), a III-V semiconducting material that has interesting electrical properties. GaSb is a material to be considered because it has a direct band gap of 0.726eV, as well as a relatively high electron mobility (≤ 3000 cm2 V-1 s-1), which makes its use possible in high speed electronic applications [1]. In this research, GaSb will be grown as a thin-film on Si substrates because the cost of pure GaSb substrates is very high relative to that of Si [2]. -
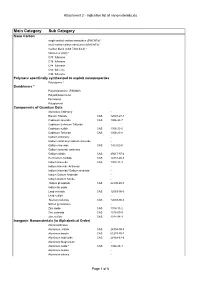
Attachment 2 - Indicative List of Nanomaterials.Xls
Attachment 2 - Indicative list of nanomaterials.xls Main Category Sub Category Nano Carbon single-walled carbon nanotubes (SWCNTs) * multi-walled carbon nanotubes (MWCNTs) * Carbon black (CAS 7440-44-0) * fullerenes (C60) * C70 fullerene C76 fullerene C84 fullerene C90 fullerene C94 fullerene Polymers specifically synthesized to exploit nanoproperties Polystyrene * Dendrimers * Polyamidoamine (PAMAM) Polyethylene imine Ferrocenyl Polyglycerol Components of Quantum Dots Aluminum Antinomy - Barium Titanate CAS 12047-27-7 Cadmium selenide CAS 1306-24-7 Cadmium Selenium Telluride Cadmium sulfide CAS 1306-23-6 Cadmium Telluride CAS 1306-25-8 Gallium Antimony - Gallium antimony/ Gallium arsenide - Gallium Arsenide CAS 1303-00-0 Gallium arsenide antimony Gallium nitride CAS 25617-97-4 Germanium carbide CAS 12334-26-8 Indium Arsenide CAS 1303-11-3 Indium Arsenide Antimony - Indium Arsenide/ Gallium arsenide - Indium Gallium Arsenide - Indium Gallium Nitride - Indium phosphide CAS 22398-80-7 Indium tin oxide - Lead selenide CAS 12069-00-0 Lead sulfide - Mercury telluride CAS 12068-90-5 Silicon germanium - Zinc oxide CAS 1314-13-2 Zinc selenide CAS 1315-09-9 Zinc sulfide CAS 1314-98-3 Inorganic Nanomaterials (in Alphabetical Order) Aluminosilicates - Aluminum nitride CAS 24304-00-5 Aluminum borate CAS 61279-70-7 Aluminum hydroxide CAS 20768-67-6 Aluminum Magnesium - Aluminum oxide * CAS 1344-28-1 Aluminum oxides - Aluminum silicate - Page 1 of 6 Attachment 2 - Indicative list of nanomaterials.xls Main Category Sub Category Aluminum Titanium Oxide -

List of Semiconductor Materials - Wikipedia, the Free Encyclopedia Page 1 of 4
List of semiconductor materials - Wikipedia, the free encyclopedia Page 1 of 4 List of semiconductor materials From Wikipedia, the free encyclopedia Semiconductor materials are insulators at absolute zero temperature that conduct electricity in a limited way at room temperature. The defining property of a semiconductor material is that it can be doped with impurities that alter its electronic properties in a controllable way. Because of their application in devices like transistors (and therefore computers) and lasers, the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. The most commonly used semiconductor materials are crystalline inorganic solids. These materials can be classified according to the periodic table groups from which their constituent atoms come. Semiconductor materials are differing by their properties. Compound semiconductors have advantages and disadvantages in comparison with silicon. For example gallium arsenide has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allows operation of power devices at higher temperatures, and gives lower thermal noise to low power devices at room temperature; its direct band gap gives it more favorable optoelectronic properties than the indirect band gap of silicon; it can be alloyed to ternary and quaternary compositions, with adjustable band gap width, allowing light emission at chosen wavelengths, and allowing e.g. matching to wavelengths with lowest losses in optical fibers. GaAs can be also grown in a semiinsulating form, which is suitable as a lattice-matching insulating substrate for GaAs devices. Conversely, silicon is robust, cheap, and easy to process, while GaAs is brittle, expensive, and insulation layers can not be created by just growing an oxide layer; GaAs is therefore used only where silicon is not sufficient.[1] Some materials can be prepared with tunable properties, e.g. -

Njit-Etd1994-008
Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. Please Note: The author retains the copyright while the New Jersey Institute of Technology reserves the right to distribute this thesis or dissertation Printing note: If you do not wish to print this page, then select “Pages from: first page # to: last page #” on the print dialog screen The Van Houten library has removed some of the personal information and all signatures from the approval page and biographical sketches of theses and dissertations in order to protect the identity of NJIT graduates and faculty. ABSTRACT Photoluminescence Study of Gallium Arsenide, Aluminum Gallium Arsenide, and Gallium Antimonide Thin Films Grown by Metalorganic Chemical Vapor Deposition by John Mark Koons The photoluminescence produced by four MOCVD grown epitaxial thin film samples was studied to give insight into sample quality. The four samples under this study were GaAs on a GaAs substrate, Al25Ga .