Endoshare: Personalizing Treatment of Endometriosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hormones and Breeding
IN-DEPTH: REPRODUCTIVE ENDOCRINOLOGY Hormones and Breeding Carlos R.F. Pinto, MedVet, PhD, Diplomate ACT Author’s address: Theriogenology and Reproductive Medicine, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210; e-mail: [email protected]. © 2013 AAEP. 1. Introduction affected by PGF treatment to induce estrus. In The administration of hormones to mares during other words, once luteolysis takes place, whether breeding management is an essential tool for equine induced by PGF treatment or occurring naturally, practitioners. Proper and timely administration of the events that follow (estrus behavior, ovulation specific hormones to broodmares may be targeted to and fertility) are essentially similar or minimally prevent reproductive disorders, to serve as an aid to affected (eg, decreased signs of behavioral estrus). treating reproductive disorders or hormonal imbal- Duration of diestrus and interovulatory intervals ances, and to optimize reproductive efficiency, for are shortened after PGF administration.1 The example, through induction of estrus or ovulation. equine corpus luteum (CL) is responsive to PGF These hormones, when administered exogenously, luteolytic effects any day after ovulation; however, act to control the duration and onset of the different only CL Ͼ5 days are responsive to one bolus injec- stages of the estrous cycle, specifically by affecting tion of PGF.2,3 Luteolysis or antiluteogenesis can duration of luteal function, hastening ovulation es- be reliably achieved in CL Ͻ5 days only if multiple pecially for timed artificial insemination and stimu- PGF treatments are administered. For that rea- lating myometrial activity in mares susceptible to or son, it became a widespread practice to administer showing delayed uterine clearance. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances" WHO/EMP/RHT/TSN/2018.1
INN Working Document 19.450 04/02/2019 Addendum1 to "The use of stems in the selection of International Nonproprietary names (INN) for pharmaceutical substances" WHO/EMP/RHT/TSN/2018.1 Programme on International Nonproprietary Names (INN) Technologies Standards and Norms (TSN) Regulation of Medicines and other health technologies (RHT) World Health Organization, Geneva © World Health Organization 2019 - All rights reserved. The contents of this document may not be reviewed, abstracted, quoted, referenced, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means, without explicit prior authorization of the WHO INN Programme. This document contains the collective views of the INN Expert Group and does not necessarily represent the decisions or the stated policy of the World Health Organization. Addendum1 to "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" - WHO/EMP/RHT/TSN/2018.1 1 This addendum is a cumulative list of all new stems selected by the INN Expert Group since the publication of "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" 2018. ------------------------------------------------------------------------------------------------------------ -calcet/-calcet- calcium-sensing receptors (CaSR) agonists cinacalcet (88), etelcalcetide (112), evocalcet (113), tecalcet (87), upacicalcet (118) ------------------------------------------------------------------------------------------------------------ -

Hertfordshire Medicines Management Committee (Hmmc) Nafarelin for Endometriosis Amber Initiation – Recommended for Restricted Use
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) NAFARELIN FOR ENDOMETRIOSIS AMBER INITIATION – RECOMMENDED FOR RESTRICTED USE Name: What it is Indication Date Decision NICE / SMC generic decision status Guidance (trade) last revised Nafarelin A potent agonistic The hormonal December Final NICE NG73 2mg/ml analogue of management of 2020 Nasal Spray gonadotrophin endometriosis, (Synarel®) releasing hormone including pain relief and (GnRH) reduction of endometriotic lesions HMMC recommendation: Amber initiation across Hertfordshire (i.e. suitable for primary care prescribing after specialist initiation) as an option in endometriosis Background Information: Gonadorelin analogues (or gonadotrophin-releasing hormone agonists [GnRHas]) include buserelin, goserelin, leuprorelin, nafarelin and triptorelin. The current HMMC decision recommends triptorelin as Decapeptyl SR® injection as the gonadorelin analogue of choice within licensed indications (which include endometriosis) link to decision. A request was made by ENHT to use nafarelin nasal spray as an alternative to triptorelin intramuscular injection during the COVID-19 pandemic. The hospital would provide initial 1 month supply, then GPs would continue for further 5 months as an alternative to the patient attending for further clinic appointments for administration of triptorelin. Previously at ENHT, triptorelin was the only gonadorelin analogue on formulary for gynaecological indications. At WHHT buserelin nasal spray 150mcg/dose is RED (hospital only) for infertility & endometriosis indications. Nafarelin nasal spray 2mg/ml is licensed for: . The hormonal management of endometriosis, including pain relief and reduction of endometriotic lesions. Use in controlled ovarian stimulation programmes prior to in-vitro fertilisation, under the supervision of an infertility specialist. Use of nafarelin in endometriosis aims to induce chronic pituitary desensitisation, which gives a menopause-like state maintained over many months. -

Myovant Sciences Ltd. 10K 2021 V1
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended March 31, 2021 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from _______ to _______ Commission file number 001-37929 Myovant Sciences Ltd. (Exact name of registrant as specified in its charter) Bermuda 98-1343578 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) Suite 1, 3rd Floor 11-12 St. James’s Square London SW1Y 4LB United Kingdom Not Applicable (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: +44 (207) 400 3351 Securities registered pursuant to Section 12(b) of the Act: Title of each Class Trading Symbol Name of each exchange on which registered Common Shares, $0.000017727 par value per share MYOV New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Obseva SA Reports Consistent Long-Term Findings from Phase 2B EDELWEISS Trial of Linzagolix for Endometriosis-Associated Pain
ObsEva SA Reports Consistent Long-Term Findings from Phase 2b EDELWEISS trial of Linzagolix for Endometriosis-Associated Pain Geneva, Switzerland and Boston, MA – May 3, 2019 – ObsEva SA (NASDAQ: OBSV / SIX: OBSN), a clinical- stage biopharmaceutical company focused on the development and commercialization of novel therapeutics for serious conditions that compromise a woman’s reproductive health and pregnancy, today reported follow-up results from the Phase 2b EDELWEISS clinical trial of its oral gonadotropin releasing hormone (GnRH) receptor antagonist, linzagolix, for the treatment of endometriosis-associated pelvic pain. These new data include 28-week extension study treatment (52 weeks of continuous treatment), as well as the 24-week post treatment follow-up (PTFU) results for patients who did not enter the extension study after completing the initial 24-week treatment period. "We are pleased to report long-term data from the EDELWEISS trial of linzagolix, which show that in patients treated with linzagolix for 52 weeks, pelvic pain response rates are maintained with the 75mg or the 200mg dose. Bone mineral density remains within safe limits. Patients that were followed for 6 months after treatment completion continue to experience pain control, and showed BMD increase,” said Ernest Loumaye, Co-Founder and Chief Executive Officer of ObsEva. “These data further support the long term therapeutic potential of linzagolix and support the currently starting Phase 3 program for the endometriosis indication, as we anticipate initial Phase 3 clinical results later this year from the trial in uterine fibroids.” Overall Pelvic Pain reduction sustained long-term After 52 weeks of treatment, responder rates for Overall Pelvic Pain (OPP)— defined as the proportion of patients experiencing an OPP score reduction >30% from baseline using a verbal rating scale— were 69% for the 75mg once daily dose and 82% for the 200mg/100mg once daily dose. -

[Product Monograph Template
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrORILISSA® elagolix (as elagolix sodium) tablets 150 mg and 200 mg Gonadotropin releasing hormone (GnRH) receptor antagonist Date of Preparation: October 4, 2018 AbbVie Corporation Date of Revision: 8401 Trans-Canada Highway March 3, 2020 St-Laurent, Qc H4S 1Z1 Submission Control No: 233793 ORILISSA Product Monograph Page 1 of 40 Date of Revision: March 3, 2020 and Control No. 233793 RECENT MAJOR LABEL CHANGES Not applicable. TABLE OF CONTENTS PART I: HEALTH PROFESSIONAL INFORMATION ............................................................... 4 1. INDICATIONS ................................................................................................................ 4 1.1. Pediatrics (< 18 years of age): .................................................................................. 4 1.2. Geriatrics (> 65 years of age): .................................................................................. 4 2. CONTRAINDICATIONS ................................................................................................. 4 3. DOSAGE AND ADMINISTRATION ................................................................................ 5 3.1. Dosing Considerations ............................................................................................. 5 3.2. Recommended Dose and Dosage Adjustment ......................................................... 5 3.3. Administration ......................................................................................................... -

Luteal Support with Very Low Daily Dose of Human Chorionic Gonadotropin After Fresh Embryo Transfer As an Alternative to Cycle S
pharmaceuticals Article Luteal Support with very Low Daily Dose of Human Chorionic Gonadotropin after Fresh Embryo Transfer as an Alternative to Cycle Segmentation for High Responders Patients Undergoing Gonadotropin-Releasing Hormone Agonist-Triggered IVF Andrea Roberto Carosso 1,*,† , Stefano Canosa 1,† , Gianluca Gennarelli 1 , Marta Sestero 1, Bernadette Evangelisti 1, Lorena Charrier 2 , Loredana Bergandi 3 , Chiara Benedetto 1,‡ and Alberto Revelli 1,‡ 1 Obstetrics and Gynecology 1U, Physiopathology of Reproduction and IVF Unit, Department of Surgical Sciences, Sant’Anna Hospital, University of Torino, 10042 Turin, Italy; [email protected] (S.C.); [email protected] (G.G.); [email protected] (M.S.); [email protected] (B.E.); [email protected] (C.B.); [email protected] (A.R.) 2 Department of Public Health and Pediatrics, University of Torino, Via Santena, 5 bis, 10126 Torino, Italy; [email protected] 3 Department of Oncology, University of Torino, Via Santena 5 bis, 10126 Torino, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-333-8111155 or +39-011-3135763 † These authors contributed equally to this work. Citation: Carosso, A.R.; Canosa, S.; ‡ These authors jointly supervised this work. Gennarelli, G.; Sestero, M.; Evangelisti, B.; Charrier, L.; Bergandi, Abstract: The segmentation of the in vitro fertilization (IVF) cycle, consisting of the freezing of L.; Benedetto, C.; Revelli, A. Luteal all embryos and the postponement of embryo transfer (ET), has become popular in recent years, Support with very Low Daily Dose of Human Chorionic Gonadotropin after with the main purpose of preventing ovarian hyperstimulation syndrome (OHSS) in patients with Fresh Embryo Transfer as an high response to controlled ovarian stimulation (COS). -

Antiprogestins, a New Form of Endocrine Therapy for Human Breast Cancer1
[CANCER RESEARCH 49, 2851-2856, June 1, 1989] Antiprogestins, a New Form of Endocrine Therapy for Human Breast Cancer1 Jan G. M. Klijn,2Frank H. de Jong, Ger H. Bakker, Steven W. J. Lamberts, Cees J. Rodenburg, and Jana Alexieva-Figusch Department of Medical Oncology (Division of Endocrine Oncology) [J. G. M. K., G. H. B., C. J. K., J. A-F.J, Dr. Daniel den Hoed Cancer Center, and Department of Endocrinology ¡F.H. d. J., S. W. ]. L.J, Erasmus University, Rotterdam, The Netherlands ABSTRACT especially pronounced effects on the endometrium, decidua, ovaries, and hypothalamo-pituitary-adrenal axis. With regard The antitumor, endocrine, hematological, biochemical, and side effects of chronic second-line treatment with the antiprogestin mifepristone (RU to clinical practice, the drug has currently been used as a contraceptive agent or abortifacient as a result of its antipro 486) were investigated in 11 postmenopausal patients with metastatic breast cancer. We observed one objective response, 6 instances of short- gestational properties (2, 22-24). Based on its antiglucocorti term stable disease, and 4 instances of progressive disease. Mean plasma coid properties, this drug has been used or has been proposed concentrations of adrenocorticotropic hormone (/' < 0.05), cortisol (/' < for treatment of conditions related to excess corticosteroid 0.001), androstenedione (/' < 0.01), and estradici (P < 0.002) increased production such as Cushing's syndrome (19, 25-27) and for significantly during treatment accompanied by a slight decrease of sex treatment of lymphomas (24) and glaucoma (28); because of its hormone binding globulin levels, while basal and stimulated gonadotropi effects on the immune system, the drug has been suggested to levels did not change significantly. -

Elagolix) Tablets, for Oral Use
Important New Update to the Prescribing Information for ORILISSA® (elagolix) tablets, for oral use In February 2021, the ORILISSA Prescribing Information (PI) was updated. The following describes several of the changes in the ORILISSA PI. Please refer to the full PI to review additional changes. The following items have been added to the Prescribing Information (PI): • Section 1 Indications and Usage – Limitations of Use: Limit the duration of use based on the dose and coexisting condition (see Table 1). • Section 4 Contraindications – With known hypersensitivity reaction to ORILISSA or any of its inactive components. Reactions have included anaphylaxis and angioedema [see Adverse Reactions (6.2)]. • Section 6 Adverse Reactions – 6.2 Postmarketing Experience The following adverse reactions have been identified during post-approval use of ORILISSA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Immune system disorders: hypersensitivity reactions (including anaphylaxis, angioedema, and urticaria). The following items have been updated in the PI to read: • Section 4 Contraindications – Taking inhibitors of organic anion transporting polypeptide (OATP) 1B1 (a hepatic uptake transporter) that are known or expected to significantly increase elagolix plasma concentrations [see Drug Interactions (7.2)]. • Section 5 Warnings and Precautions – 5.5 Interactions with Hormonal Contraceptives Advise -

EC313-A Tissue Selective SPRM Reduces the Growth and Proliferation of Uterine Fbroids in a Human Uterine Fbroid Tissue Xenograft Model Hareesh B
www.nature.com/scientificreports OPEN EC313-a tissue selective SPRM reduces the growth and proliferation of uterine fbroids in a human uterine fbroid tissue xenograft model Hareesh B. Nair1*, Bindu Santhamma1, Kalarickal V. Dileep2, Peter Binkley3, Kirk Acosta1, Kam Y. J. Zhang 2, Robert Schenken3 & Klaus Nickisch1 Uterine fbroids (UFs) are associated with irregular or excessive uterine bleeding, pelvic pain or pressure, or infertility. Ovarian steroid hormones support the growth and maintenance of UFs. Ulipristal acetate (UPA) a selective progesterone receptor (PR) modulator (SPRM) reduce the size of UFs, inhibit ovulation and lead to amenorrhea. Recent liver toxicity concerns with UPA, diminished enthusiasm for its use and reinstate the critical need for a safe, efcacious SPRM to treat UFs. In the current study, we evaluated the efcacy of new SPRM, EC313, for the treatment for UFs using a NOD-SCID mouse model. EC313 treatment resulted in a dose-dependent reduction in the fbroid xenograft weight (p < 0.01). Estradiol (E2) induced proliferation was blocked signifcantly in EC313-treated xenograft fbroids (p < 0.0001). Uterine weight was reduced by EC313 treatment compared to UPA treatment. ER and PR were reduced in EC313-treated groups compared to controls (p < 0.001) and UPA treatments (p < 0.01). UF specifc desmin and collagen were markedly reduced with EC313 treatment. The partial PR agonism and no signs of unopposed estrogenicity makes EC313 a candidate for the long-term treatment for UFs. Docking studies have provided a structure based explanation for the SPRM activity of EC313. Te unmet need for medical management of uterine fbroids (UFs) has led to the discovery of various novel agents in recent years. -

Clinical Study Protocol M12-815 a Phase 3 Study to Evaluate The
NCT02654054 Elagolix (ABT-620) M12-815 Protocol Amendment 3 1.0 Title Page Clinical Study Protocol M12-815 A Phase 3 Study to Evaluate the Efficacy and Safety of Elagolix in Combination with Estradiol/Norethindrone Acetate for the Management of Heavy Menstrual Bleeding Associated with Uterine Fibroids in Premenopausal Women Incorporating Amendments 1, 2 and 3 AbbVie Investigational Product: Elagolix (ABT-620) Date: 25 September 2017 Development Phase: 3 Study Design: Phase 3, randomized, double-blind, multicenter, placebo-controlled study evaluating the efficacy, safety and tolerability of elagolix alone and in combination with estradiol/norethindrone acetate for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women 18 to 51 years of age. Investigators: Multicenter Trial: Investigator information is on file at AbbVie Sponsor: AbbVie Sponsor/Emergency Contact: This study will be conducted in compliance with the protocol, Good Clinical Practice and all other applicable regulatory requirements, including the archiving of essential documents. Confidential Information No use or disclosure outside AbbVie is permitted without prior written authorization from AbbVie. 1 Elagolix (ABT-620) M12-815 Protocol Amendment 3 1.1 Protocol Amendment: Summary of Changes Previous Protocol Versions Protocol Date Original 06 November 2015 Amendment 1 01 December 2015 Amendment 2 23 June 2016 The purpose of this Amendment is to: ● Update Section 1.1 Protocol Amendment: Summary of Changes from Appendix Q to Appendix -
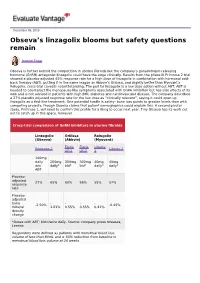
Obseva's Linzagolix Blooms but Safety Questions Remain
December 09, 2019 Obseva’s linzagolix blooms but safety questions remain Joanne Fagg Obseva is further behind the competition in uterine fibroids but the company’s gonadotropin-releasing hormone (GnRH) antagonist linzagolix could have the edge clinically. Results from the phase III Primrose 2 trial showed a placebo-adjusted 65% response rate for a high dose of linzagolix in combination with hormonal add- back therapy (ABT), putting it in the same league as Abbvie’s Orilissa, and slightly better than Myovant’s Relugolix, cross-trial caveats notwithstanding. The pull for linzagolix is a low dose option without ABT; ABT is needed to counteract the menopause-like symptoms associated with GnRH inhibition but has side effects of its own and is not advised in patients with high BMI, diabetes and cardiovascular disease. The company described a 27% placebo adjusted response rate for the low dose as “clinically relevant”, saying it could open up linzagolix as a first-line treatment. One potential hurdle is safety: bone loss points to greater levels than with competing projects, though Obseva claims that patient demographics could explain this. A second pivotal study, Primrose 1, will need to confirm this profile for a filing to occur next year. Tiny Obseva has its work cut out to catch up in this space, however. Cross-trial comparison of GnRH inhibitors in uterine fibroids Linzagolix Orilissa Relugolix (Obseva) (Abbvie) (Myovant) Elaris Elaris Liberty Primrose 2 Liberty 2 UF-1 UF-2 1 100mg daily 200mg 300mg 300mg 40mg 40mg w/o daily* bid* bid* daily* daily* ABT Placebo- adjusted 27% 65% 60% 66% 55% 57% response rate Placebo- adjusted bone - - - - -2.50% -0.45% mineral 1.81% 0.55% 0.55% 0.41% density change *Doses with ABT; bid=twice daily.