The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances" WHO/EMP/RHT/TSN/2018.1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Myovant Sciences Ltd. 10K 2021 V1
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended March 31, 2021 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from _______ to _______ Commission file number 001-37929 Myovant Sciences Ltd. (Exact name of registrant as specified in its charter) Bermuda 98-1343578 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) Suite 1, 3rd Floor 11-12 St. James’s Square London SW1Y 4LB United Kingdom Not Applicable (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: +44 (207) 400 3351 Securities registered pursuant to Section 12(b) of the Act: Title of each Class Trading Symbol Name of each exchange on which registered Common Shares, $0.000017727 par value per share MYOV New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Obseva SA Reports Consistent Long-Term Findings from Phase 2B EDELWEISS Trial of Linzagolix for Endometriosis-Associated Pain
ObsEva SA Reports Consistent Long-Term Findings from Phase 2b EDELWEISS trial of Linzagolix for Endometriosis-Associated Pain Geneva, Switzerland and Boston, MA – May 3, 2019 – ObsEva SA (NASDAQ: OBSV / SIX: OBSN), a clinical- stage biopharmaceutical company focused on the development and commercialization of novel therapeutics for serious conditions that compromise a woman’s reproductive health and pregnancy, today reported follow-up results from the Phase 2b EDELWEISS clinical trial of its oral gonadotropin releasing hormone (GnRH) receptor antagonist, linzagolix, for the treatment of endometriosis-associated pelvic pain. These new data include 28-week extension study treatment (52 weeks of continuous treatment), as well as the 24-week post treatment follow-up (PTFU) results for patients who did not enter the extension study after completing the initial 24-week treatment period. "We are pleased to report long-term data from the EDELWEISS trial of linzagolix, which show that in patients treated with linzagolix for 52 weeks, pelvic pain response rates are maintained with the 75mg or the 200mg dose. Bone mineral density remains within safe limits. Patients that were followed for 6 months after treatment completion continue to experience pain control, and showed BMD increase,” said Ernest Loumaye, Co-Founder and Chief Executive Officer of ObsEva. “These data further support the long term therapeutic potential of linzagolix and support the currently starting Phase 3 program for the endometriosis indication, as we anticipate initial Phase 3 clinical results later this year from the trial in uterine fibroids.” Overall Pelvic Pain reduction sustained long-term After 52 weeks of treatment, responder rates for Overall Pelvic Pain (OPP)— defined as the proportion of patients experiencing an OPP score reduction >30% from baseline using a verbal rating scale— were 69% for the 75mg once daily dose and 82% for the 200mg/100mg once daily dose. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau Et Al
US 20150202317A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau et al. (43) Pub. Date: Jul. 23, 2015 (54) DIPEPTDE-BASED PRODRUG LINKERS Publication Classification FOR ALPHATIC AMNE-CONTAINING DRUGS (51) Int. Cl. A647/48 (2006.01) (71) Applicant: Ascendis Pharma A/S, Hellerup (DK) A638/26 (2006.01) A6M5/9 (2006.01) (72) Inventors: Harald Rau, Heidelberg (DE); Torben A 6LX3/553 (2006.01) Le?mann, Neustadt an der Weinstrasse (52) U.S. Cl. (DE) CPC ......... A61K 47/48338 (2013.01); A61 K3I/553 (2013.01); A61 K38/26 (2013.01); A61 K (21) Appl. No.: 14/674,928 47/48215 (2013.01); A61M 5/19 (2013.01) (22) Filed: Mar. 31, 2015 (57) ABSTRACT The present invention relates to a prodrug or a pharmaceuti Related U.S. Application Data cally acceptable salt thereof, comprising a drug linker conju (63) Continuation of application No. 13/574,092, filed on gate D-L, wherein D being a biologically active moiety con Oct. 15, 2012, filed as application No. PCT/EP2011/ taining an aliphatic amine group is conjugated to one or more 050821 on Jan. 21, 2011. polymeric carriers via dipeptide-containing linkers L. Such carrier-linked prodrugs achieve drug releases with therapeu (30) Foreign Application Priority Data tically useful half-lives. The invention also relates to pharma ceutical compositions comprising said prodrugs and their use Jan. 22, 2010 (EP) ................................ 10 151564.1 as medicaments. US 2015/0202317 A1 Jul. 23, 2015 DIPEPTDE-BASED PRODRUG LINKERS 0007 Alternatively, the drugs may be conjugated to a car FOR ALPHATIC AMNE-CONTAINING rier through permanent covalent bonds. -

Recent Development of Non-Peptide Gnrh Antagonists
Review Recent Development of Non-Peptide GnRH Antagonists Feng-Ling Tukun 1, Dag Erlend Olberg 1,2, Patrick J. Riss 2,3,4, Ira Haraldsen 4, Anita Kaass 5 and Jo Klaveness 1,* 1 School of Pharmacy, University of Oslo, 0316 Oslo, Norway; [email protected] (F.-L.T.); [email protected] (D.E.O.) 2 Norsk Medisinsk Syklotronsenter AS, Postboks 4950 Nydalen, 0424 Oslo, Norway; [email protected] 3 Realomics SFI, Department of Chemistry, University of Oslo, 0316 Oslo, Norway 4 Department of neuropsychiatry and psychosomatic medicine, Oslo University Hospital, 4950 Oslo, Norway; [email protected] 5 Betanien Hospital, 3722 Skien, Norway; [email protected] * Correspondence: [email protected]; Tel.: +47-9177-6204 Received: 16 November 2017; Accepted: 4 December 2017; Published: 9 December 2017 Abstract: The decapeptide gonadotropin-releasing hormone, also referred to as luteinizing hormone-releasing hormone with the sequence (pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2) plays an important role in regulating the reproductive system. It stimulates differential release of the gonadotropins FSH and LH from pituitary tissue. To date, treatment of hormone-dependent diseases targeting the GnRH receptor, including peptide GnRH agonist and antagonists are now available on the market. The inherited issues associate with peptide agonists and antagonists have however, led to significant interest in developing orally active, small molecule, non-peptide antagonists. In this review, we will summarize all developed small molecule GnRH antagonists along with the most recent clinical data and therapeutic applications. Keywords: GnRH receptor; non-peptide GnRH antagonist 1. -

EC313-A Tissue Selective SPRM Reduces the Growth and Proliferation of Uterine Fbroids in a Human Uterine Fbroid Tissue Xenograft Model Hareesh B
www.nature.com/scientificreports OPEN EC313-a tissue selective SPRM reduces the growth and proliferation of uterine fbroids in a human uterine fbroid tissue xenograft model Hareesh B. Nair1*, Bindu Santhamma1, Kalarickal V. Dileep2, Peter Binkley3, Kirk Acosta1, Kam Y. J. Zhang 2, Robert Schenken3 & Klaus Nickisch1 Uterine fbroids (UFs) are associated with irregular or excessive uterine bleeding, pelvic pain or pressure, or infertility. Ovarian steroid hormones support the growth and maintenance of UFs. Ulipristal acetate (UPA) a selective progesterone receptor (PR) modulator (SPRM) reduce the size of UFs, inhibit ovulation and lead to amenorrhea. Recent liver toxicity concerns with UPA, diminished enthusiasm for its use and reinstate the critical need for a safe, efcacious SPRM to treat UFs. In the current study, we evaluated the efcacy of new SPRM, EC313, for the treatment for UFs using a NOD-SCID mouse model. EC313 treatment resulted in a dose-dependent reduction in the fbroid xenograft weight (p < 0.01). Estradiol (E2) induced proliferation was blocked signifcantly in EC313-treated xenograft fbroids (p < 0.0001). Uterine weight was reduced by EC313 treatment compared to UPA treatment. ER and PR were reduced in EC313-treated groups compared to controls (p < 0.001) and UPA treatments (p < 0.01). UF specifc desmin and collagen were markedly reduced with EC313 treatment. The partial PR agonism and no signs of unopposed estrogenicity makes EC313 a candidate for the long-term treatment for UFs. Docking studies have provided a structure based explanation for the SPRM activity of EC313. Te unmet need for medical management of uterine fbroids (UFs) has led to the discovery of various novel agents in recent years. -

First Principles and Their Application to Drug Discovery
REVIEWS Drug Discovery Today Volume 17, Numbers 1/2 January 2012 The utilization of the kinetic and thermodynamic signatures of preclinical leads is proving pivotal in their triage and rational optimization towards clinical candidates with maximal in vivo efficacy devoid of adverse events. Reviews KEYNOTE REVIEW Target–drug interactions: first principles and their application to drug discovery 1 1 Sara Nu´n˜ez studied organic Sara Nu´n˜ ez , Jennifer Venhorst and Chris G. Kruse chemistry at the University of Barcelona (Spain) and the Abbott Healthcare Products, 1381 CP Weesp, The Netherlands University of London (UK). She received her Ph.D. in 2003 from the University of Manchester (UK), and thereafter did a In this review, we begin by introducing the basic principles of kinetics postdoc in Biophysics at the and thermodynamics of target–drug binding within the context of Albert Einstein College of Medicine (USA). In 2005, she drug discovery. In addition, we present a meta-analysis of the recent joined Solvay Pharmaceuticals (now Abbott Healthcare) in The Netherlands as a postdoctoral fellow; and in 2008, she literature describing the kinetic and thermodynamic resolution of was promoted to Sr. Computational Medicinal Chemist. At Abbott, she has supported the medicinal chemistry efforts successful clinical candidates with diverse mechanisms of action. for neuroscience drug discovery programs, from target We finish by discussing the best practices in the triage and chemical discovery up to and including clinical proof of principle studies. She has supported more than 15 programs optimization towards clinical candidates with maximal in vivo internationally, and was project manager of the D2-103 Top Institute Pharma innitiative. -
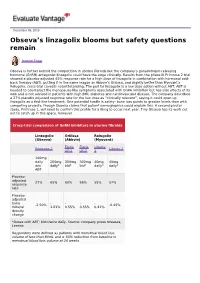
Obseva's Linzagolix Blooms but Safety Questions Remain
December 09, 2019 Obseva’s linzagolix blooms but safety questions remain Joanne Fagg Obseva is further behind the competition in uterine fibroids but the company’s gonadotropin-releasing hormone (GnRH) antagonist linzagolix could have the edge clinically. Results from the phase III Primrose 2 trial showed a placebo-adjusted 65% response rate for a high dose of linzagolix in combination with hormonal add- back therapy (ABT), putting it in the same league as Abbvie’s Orilissa, and slightly better than Myovant’s Relugolix, cross-trial caveats notwithstanding. The pull for linzagolix is a low dose option without ABT; ABT is needed to counteract the menopause-like symptoms associated with GnRH inhibition but has side effects of its own and is not advised in patients with high BMI, diabetes and cardiovascular disease. The company described a 27% placebo adjusted response rate for the low dose as “clinically relevant”, saying it could open up linzagolix as a first-line treatment. One potential hurdle is safety: bone loss points to greater levels than with competing projects, though Obseva claims that patient demographics could explain this. A second pivotal study, Primrose 1, will need to confirm this profile for a filing to occur next year. Tiny Obseva has its work cut out to catch up in this space, however. Cross-trial comparison of GnRH inhibitors in uterine fibroids Linzagolix Orilissa Relugolix (Obseva) (Abbvie) (Myovant) Elaris Elaris Liberty Primrose 2 Liberty 2 UF-1 UF-2 1 100mg daily 200mg 300mg 300mg 40mg 40mg w/o daily* bid* bid* daily* daily* ABT Placebo- adjusted 27% 65% 60% 66% 55% 57% response rate Placebo- adjusted bone - - - - -2.50% -0.45% mineral 1.81% 0.55% 0.55% 0.41% density change *Doses with ABT; bid=twice daily. -

WO 2011/089216 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date t 28 July 2011 (28.07.2011) WO 2011/089216 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/48 (2006.01) C07K 1/13 (2006.01) kind of national protection available): AE, AG, AL, AM, C07K 1/1 07 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) Number: International Application DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/050821 HN, HR, HU, ID, J , IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 2 1 January 201 1 (21 .01 .201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 1015 1465. 1 22 January 2010 (22.01 .2010) EP kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): AS- ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, CENDIS PHARMA AS [DK/DK]; Tuborg Boulevard TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 12, DK-2900 Hellerup (DK). -

Obseva Needs More to Beat Abbvie in Endometriosis
June 19, 2018 Obseva needs more to beat Abbvie in endometriosis Madeleine Armstrong Obseva, lagging behind its bigger rival Abbvie in the oral gonadotrophin-releasing hormone antagonist space, has a clear mission. The smaller company’s candidate, linzagolix, will need to show best-in-class efficacy if it is to have a chance of competing with Abbvie’s elagolix, which could hit the market this year in its first indication, endometriosis-associated pain. Obseva believes that it has gone some way to proving linzagolix’s superiority with data from the small phase IIb Edelweiss trial in the same disorder, and investors sent the group’s stock up 23% yesterday in response. But a look at how the results stack up against data from elagolix’s pivotal trials suggests some cause for concern (see table below). Of course, cross-trial comparisons should always be treated with caution, and this one is particularly fraught with difficulty, as the studies used different endpoints. But on the most similar measure, response on non- menstrual pain, linzagolix appears to fall short, particularly at the highest dose used, 200mg. Cross-trial comparison of linzagolix and elagolix Placebo 50mg 75mg 100mg 150mg 200mg Linzagolix/OBE2109: Edelweiss (phase IIb, NCT02778399) Response rate (primary endpoint)* 35% 49% 62% 56% - 56% P value - 0.155 0.003 0.039 - 0.034 Response rate, nonmenstrual pain 37% 46% 59% 62% - 48% P value - 0.38 0.017 0.022 - 0.297 Elagolix: Elaris EM-I, Elaris EM-II (phase III, NCT01620528, NCT01931670) Response rate, nonmenstrual pain 37% - - - 50%** 55-58%** *Response defined as reduction of ≥30% in combined menstrual and non-menstrual pelvic pain at week 12; **p<0.003; Source: Company presentations; NEJM paper. -

INN Working Document 20.472 09/03/2020
INN Working Document 20.472 09/03/2020 Addendum1 to "The use of stems in the selection of International Nonproprietary names (INN) for pharmaceutical substances" WHO/EMP/RHT/TSN/2018.1 Programme on International Nonproprietary Names (INN) Medicines and Health Products World Health Organization, Geneva © World Health Organization 2020 - All rights reserved. The contents of this document may not be reviewed, abstracted, quoted, referenced, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means, without explicit prior authorization of the WHO INN Programme. This document contains the collective views of the INN Expert Group and does not necessarily represent the decisions or the stated policy of the World Health Organization. Addendum1 to "The use of stem s in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" - WHO/EMP/RHT/TSN/2018.1 1 This addendum is a cumulative list of all new stems selected by the INN Expert Group since the publication of "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" 2018. -caftor cystic fibrosis transmembrane regulator (CFTR) protein modulators, correctors, and amplifiers bamocaftor (121), deutivacaftor (118), elexacaftor (121), galicaftor (119), icenticaftor (122), ivacaftor (104), lumacaftor (105), navocaftor (121), nesolicaftor (122), olacaftor (119), posenacaftor (122), tezacaftor (114) -calcet/-calcet- calcium-sensing receptors (CaSR) agonists cinacalcet -

(12) United States Patent (10) Patent No.: US 8,158,152 B2 Palepu (45) Date of Patent: Apr
US008158152B2 (12) United States Patent (10) Patent No.: US 8,158,152 B2 Palepu (45) Date of Patent: Apr. 17, 2012 (54) LYOPHILIZATION PROCESS AND 6,884,422 B1 4/2005 Liu et al. PRODUCTS OBTANED THEREBY 6,900, 184 B2 5/2005 Cohen et al. 2002fOO 10357 A1 1/2002 Stogniew etal. 2002/009 1270 A1 7, 2002 Wu et al. (75) Inventor: Nageswara R. Palepu. Mill Creek, WA 2002/0143038 A1 10/2002 Bandyopadhyay et al. (US) 2002fO155097 A1 10, 2002 Te 2003, OO68416 A1 4/2003 Burgess et al. 2003/0077321 A1 4/2003 Kiel et al. (73) Assignee: SciDose LLC, Amherst, MA (US) 2003, OO82236 A1 5/2003 Mathiowitz et al. 2003/0096378 A1 5/2003 Qiu et al. (*) Notice: Subject to any disclaimer, the term of this 2003/OO96797 A1 5/2003 Stogniew et al. patent is extended or adjusted under 35 2003.01.1331.6 A1 6/2003 Kaisheva et al. U.S.C. 154(b) by 1560 days. 2003. O191157 A1 10, 2003 Doen 2003/0202978 A1 10, 2003 Maa et al. 2003/0211042 A1 11/2003 Evans (21) Appl. No.: 11/282,507 2003/0229027 A1 12/2003 Eissens et al. 2004.0005351 A1 1/2004 Kwon (22) Filed: Nov. 18, 2005 2004/0042971 A1 3/2004 Truong-Le et al. 2004/0042972 A1 3/2004 Truong-Le et al. (65) Prior Publication Data 2004.0043042 A1 3/2004 Johnson et al. 2004/OO57927 A1 3/2004 Warne et al. US 2007/O116729 A1 May 24, 2007 2004, OO63792 A1 4/2004 Khera et al.