Hormones and Breeding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Costs for Treating Central Precocious Puberty During the First Year with Monthly Leuprolide Acetate Injectable and Histrelin Acetate Implant
A Comparison Of The Costs For Treating Central Precocious Puberty During The First Year With Monthly Leuprolide Acetate Injectable And Histrelin Acetate Implant B F Banahan III1, Mayo K2, Summers KH2 1 Center for Pharmaceutical Marketing and Management and Department of Pharmacy Administration, University of Mississippi, University, MS 2 Health Outcomes & PharmacoEconomics, Endo Pharmaceuticals, Inc., Chadds Ford, PA Estimated Annual Cost of Treatment if all PTs Treated With . BACKGROUND METHODS MEDICAID MODEL Lupron SUPPRELIN LA Product costs $ 1,770,201 $ 1,515,188 Two retrospective cohort studies were conducted using datasets derived from the Office visit costs $ 79,896 $ 14,000 Puberty results when secretion of gonadotropin releasing hormone (GnRH) is initiated 100% compliance with Lupron treatment 1 Implant procedures $ 91,400 and the hypothalamic-pituitary-gonadal axis is activated. During puberty, the brain Thomas Reuter’s MarketScan© Multi-State Medicaid Database (2003-2007) and the (All patients receive 13+ treatments in year) MarketScan© Commercial Database (2005-2009). A probabilistic patient flow model Lab/xray for monitoring $ 53,900 $ 37,500 produces GnRH through a complex process. GnRH causes increases in other TOTAL COST TO PAYER $ 1,903,997 $ 1,658,088 hormones like luteinizing hormone (LH) and follicle stimulating hormone (FSH). It is was developed using estimates for treatment patterns and costs for products, office Normal Compliance with Lupron: Lupron SUPPRELIN LA these hormones that cause the ovaries to produce estrogen and the testicles to visits, and monitoring therapy. Patients with < 13 treatments per year Product costs $ 1,572,921 $ 1,515,188 Quality of Care ofCare Quality % of TXd PTs: 53% Aver. -

Download Article As
FOCUS > EQUINE FOCUS < OVINE FOCUS > EQUINE Inducing timed ovulation in the mare Susan Salter BSc Hons BVM&S MRCVS and Jonathon Pycock BVetMed PhD DESM MRCVS compare and contrast various ovulating agents used to induce ovulation in mares at breeding, highlighting the advantages and disadvantages, effi cacy and welfare implications associated with each Weatherbys documented 14,747 active thoroughbred hCG was used on subsequent cycles. They also showed broodmares in Ireland in 2019, almost twice as many as that younger mares were more likely to ovulate within 48 the UK which recorded 8,571. Ireland is the third biggest hours than older mares when given hCG. Repeated use of producer of thoroughbreds in the world after Australia and hCG is, therefore, associated with decreased reliability in the USA.1 inducing timed ovulation and e icacy declines significantly In addition, it is estimated that there are around 15,000 active with increased mare age making it unreliable for use in breeders in the sport horse sector. 2 In order to maximise the older mares.3 In Ireland, hCG is still used since the deslorelin e iciency of breeding, it is essential that timing of ovulation implant has labour, cost, welfare and safety implications. can be manipulated e ectively. It is also imperative that attempts to manipulate timing of ovulation are not associated DESLORELIN ACETATE – THE IMPLANT AND THE with subsequent delays in return to oestrus. The current INJECTABLE Covid-19 crisis presents additional challenges and pressures Deslorelin is a gonadotrophin releasing hormone (GnRH) of balancing the economic imperative to continue equine receptor agonist. -

Hertfordshire Medicines Management Committee (Hmmc) Nafarelin for Endometriosis Amber Initiation – Recommended for Restricted Use
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) NAFARELIN FOR ENDOMETRIOSIS AMBER INITIATION – RECOMMENDED FOR RESTRICTED USE Name: What it is Indication Date Decision NICE / SMC generic decision status Guidance (trade) last revised Nafarelin A potent agonistic The hormonal December Final NICE NG73 2mg/ml analogue of management of 2020 Nasal Spray gonadotrophin endometriosis, (Synarel®) releasing hormone including pain relief and (GnRH) reduction of endometriotic lesions HMMC recommendation: Amber initiation across Hertfordshire (i.e. suitable for primary care prescribing after specialist initiation) as an option in endometriosis Background Information: Gonadorelin analogues (or gonadotrophin-releasing hormone agonists [GnRHas]) include buserelin, goserelin, leuprorelin, nafarelin and triptorelin. The current HMMC decision recommends triptorelin as Decapeptyl SR® injection as the gonadorelin analogue of choice within licensed indications (which include endometriosis) link to decision. A request was made by ENHT to use nafarelin nasal spray as an alternative to triptorelin intramuscular injection during the COVID-19 pandemic. The hospital would provide initial 1 month supply, then GPs would continue for further 5 months as an alternative to the patient attending for further clinic appointments for administration of triptorelin. Previously at ENHT, triptorelin was the only gonadorelin analogue on formulary for gynaecological indications. At WHHT buserelin nasal spray 150mcg/dose is RED (hospital only) for infertility & endometriosis indications. Nafarelin nasal spray 2mg/ml is licensed for: . The hormonal management of endometriosis, including pain relief and reduction of endometriotic lesions. Use in controlled ovarian stimulation programmes prior to in-vitro fertilisation, under the supervision of an infertility specialist. Use of nafarelin in endometriosis aims to induce chronic pituitary desensitisation, which gives a menopause-like state maintained over many months. -

Antiluteogenic Effects of Serial Prostaglandin F2α Administration in Mares
ANTILUTEOGENIC EFFECTS OF SERIAL PROSTAGLANDIN F2α ADMINISTRATION IN MARES Thesis Presented in partial fulfillment of the requirements for the Master of Science in the Graduate School of The Ohio State University Elizabeth Ann Coffman Graduate ProGram in Comparative and Veterinary Medicine The Ohio State University 2013 Thesis committee: Carlos R.F. Pinto PhD, MV, DACT; Dissertation Advisor Marco A. Coutinho da Silva PhD, MV, DACT; Academic Advisor Christopher Premanandan PhD, DVM, DACVP Copyright by Elizabeth Ann Coffman 2013 Abstract For breedinG manaGement and estrus synchronization, prostaGlandin F2α (PGF) is one of the most commonly utilized hormones to pharmacologically manipulate the equine estrous cycle. There is a general supposition a sinGle dose of PGF does not consistently induce luteolysis in the equine corpus luteum (CL) until at least five to six days after ovulation. This leads to the erroneous assumption that the early CL (before day five after ovulation) is refractory to the luteolytic effects of PGF. An experiment was desiGned to test the hypotheses that serial administration of PGF in early diestrus would induce a return to estrus similar to mares treated with a sinGle injection in mid diestrus, and fertility of the induced estrus for the two treatment groups would not differ. The specific objectives of the study were to evaluate the effects of early diestrus treatment by: 1) assessing the luteal function as reflected by hormone profile for concentration of plasma progesterone; 2) determininG the duration of interovulatory and treatment to ovulation intervals; 3) comparing of the number of pregnant mares at 14 days post- ovulation. The study consisted of a balanced crossover desiGn in which reproductively normal Quarter horse mares (n=10) were exposed to two treatments ii on consecutive reproductive cycles. -

(CERCA), Ecole Nationale Vétérinaire D'alfort
Rev. Bras. Reprod. Anim., Belo Horizonte, v.35, n.2, p.210-216, abr./jun. 2011. Disponível em ww.cbra.org.br. The use of GnRH agonists implants in bitches and queens Utilização de implantes de agonistas do GnRH em cadelas e gatas A. Fontbonne1, E. Fontaine Centre d’Etude en Reproduction des Carnivores (CERCA), Alfort Veterinary College, Paris, France. 1Corresponding author: [email protected] Abstract GnRH (gonadotrophin releasing hormone) is a key hormone of reproductive function in mammals; agonist forms have been largely developed, and data concerning their use in small animal reproduction are now abundant. GnRH agonists act by a two-step mechanism. First, their agonist properties on the pituitary will cause marked LH (luteinizing hormone) and FSH (follicle-stimulating hormone) secretion into the bloodstream, accompanied by an increase in the concentrations of sex steroid hormones. Then, in case of constant administration, GnRH agonists will lead to pituitary desensitization, and FSH and LH levels will collapse. These two effects have been widely documented, and these compounds have many potential benefits in a clinical context, capitalizing both on their stimulating and sterilizing effects. Keywords: bitch, deslorelin, GnRH agonists, queen. Resumo O hormônio liberador de gonadotrofinas (GnRH) é um hormônio chave na função reprodutiva dos mamíferos. Formas agonistas têm sido amplamente desenvolvidas e atualmente existem muitas informações sobre sua utilização na reprodução de pequenos animais. Os agonistas do GnRH atuam por meio de um mecanismo que envolve duas etapas. Inicialmente, suas propriedades agonistas irão causar secreção marcante de hormônio luteinizante (LH) e folículo-estimulante (FSH) pela hipófise, acompanhado pelo aumento das concentrações dos hormônios esteróides sexuais. -

Use of a Gonadotropin Releasing Hormone Agonist Implant
Use of a Gonadotropin Releasing Hormone Agonist Implant Containing 4.7 mg Deslorelin for Medical Castration in Male Ferrets (Mustela putorius furo) Bulliot Christophe, DVM1 Mentré Véronique, DVM2 Berthelet Adeline, DVM3 Navarro Christelle, DVM4 Bidaud Alice, DVM4 1 Corresponding author: Clinique Vétérinaire Exotic Clinic, 38 rue Robert Cousin, 77176 Nandy, France. E-mail: [email protected] 2 Clinique Vétérinaire de la Patte d’Oie, 155 Bd Victor Bordier, 95370 Montigny les Cormeilles, France. E-mail: [email protected] 3 Clinique Vétérinaire Exotic Clinic, 38 rue Robert Cousin, 77176 Nandy, France. E-mail: [email protected] 4 Virbac, 13ème rue - LID, 06511 Carros, France. Conflict of interest: the study was funded by Virbac. KEY WORDS: Ferret, Deslorelin, tent, for medical castration. This study is the Gonadotropin, GnRH, medical castration first evaluation of a GnRH-agonist implant containing 4.7 mg deslorelin for medical ABSTRACT castration in male ferrets with an assessment Sterilization of ferrets (Mustela putorius of the duration of infertility over a 3-year furo) is a common practice. Male ferrets, follow-up. Twenty-nine intact male ferrets unlike females, don’t need castration for in rut were implanted and were used for medical reasons, but are frequently neutered tolerance evaluation. Infertility was assessed to prevent reproduction and reduce their in 27 ferrets by evaluating their testosterone musky odor and aggressive territorial behav- concentrations, testis size and musky odor. ior. The search for an alternative to surgical Our results indicated that infertility was in- castration is an important goal and challenge duced within 6 weeks post-implantation and in this species. -

DESCRIPTION Vantas™ (Histrelin Implant) Is a Sterile Non-Biodegradable, Diffusion-Controlled Reservoir Drug Delivery System De
Vantas™ (histrelin implant) PACKAGE INSERT DESCRIPTION Vantas™ (histrelin implant) is a sterile non-biodegradable, diffusion-controlled reservoir drug delivery system designed to deliver histrelin continuously for 12 months upon subcutaneous implantation. The Vantas implant contains 50 mg of histrelin acetate. Histrelin acetate is a synthetic nonapeptide analogue of the naturally occurring gonadotropin releasing hormone (GnRH) or luteinizing hormone releasing hormone (LH-RH). The sterile Vantas implantation device (provided with the implant) is used to insert the implant subcutaneously in the inner aspect of the upper arm. After 12 months, the implant must be removed. At the time the implant is removed, another implant may be inserted to continue therapy. The sterile Vantas ™ implant consists of a 50-mg histrelin acetate drug core inside a non- biodegradable, 3 cm by 3.5 mm cylindrically shaped hydrogel reservoir (Figure A). The drug core also contains the inactive ingredient stearic acid NF. The hydrogel reservoir is a hydrophilic polymer cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. The hydrated implant is packaged in a glass vial containing 2.0 mL of 1.8% NaCl solution. The implant is primed for release of the drug upon insertion. Figure A. Vantas Histrelin Implant diagram (not to scale) Hydrogel Reservoir Drug Formulation Histrelin acetate is chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L- tyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) [C66H86N18O12. (1.7-2.8 moles) CH3COOH, (0.6-7.0 moles) H2O], with the molecular weight of 1443.70 (or 1323.50 as histrelin base. -

Luteal Support with Very Low Daily Dose of Human Chorionic Gonadotropin After Fresh Embryo Transfer As an Alternative to Cycle S
pharmaceuticals Article Luteal Support with very Low Daily Dose of Human Chorionic Gonadotropin after Fresh Embryo Transfer as an Alternative to Cycle Segmentation for High Responders Patients Undergoing Gonadotropin-Releasing Hormone Agonist-Triggered IVF Andrea Roberto Carosso 1,*,† , Stefano Canosa 1,† , Gianluca Gennarelli 1 , Marta Sestero 1, Bernadette Evangelisti 1, Lorena Charrier 2 , Loredana Bergandi 3 , Chiara Benedetto 1,‡ and Alberto Revelli 1,‡ 1 Obstetrics and Gynecology 1U, Physiopathology of Reproduction and IVF Unit, Department of Surgical Sciences, Sant’Anna Hospital, University of Torino, 10042 Turin, Italy; [email protected] (S.C.); [email protected] (G.G.); [email protected] (M.S.); [email protected] (B.E.); [email protected] (C.B.); [email protected] (A.R.) 2 Department of Public Health and Pediatrics, University of Torino, Via Santena, 5 bis, 10126 Torino, Italy; [email protected] 3 Department of Oncology, University of Torino, Via Santena 5 bis, 10126 Torino, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-333-8111155 or +39-011-3135763 † These authors contributed equally to this work. Citation: Carosso, A.R.; Canosa, S.; ‡ These authors jointly supervised this work. Gennarelli, G.; Sestero, M.; Evangelisti, B.; Charrier, L.; Bergandi, Abstract: The segmentation of the in vitro fertilization (IVF) cycle, consisting of the freezing of L.; Benedetto, C.; Revelli, A. Luteal all embryos and the postponement of embryo transfer (ET), has become popular in recent years, Support with very Low Daily Dose of Human Chorionic Gonadotropin after with the main purpose of preventing ovarian hyperstimulation syndrome (OHSS) in patients with Fresh Embryo Transfer as an high response to controlled ovarian stimulation (COS). -

2018 Summary of Major Modifications and Explanatory Notes
SUMMARY OF MAJOR MODIFICATIONS AND EXPLANATORY NOTES 2018 PROHIBITED LIST Substances and methods prohibited at all times (In- and Out-of-Competition) Prohibited Substances S1 ANABOLIC AGENTS S3 BETA-2-AGONISTS • Dihydrotestosterone was renamed to its International • Dosing parameters of salbutamol were revised to make Non-proprietary Name (INN) (androstanolone). it clear that divided doses of salbutamol may not exceed 1-androsterone (3α-hydroxy-5α-androst-1-ene-17-one) 800 micrograms over any 12 hours (see figure). was added in S1.a as an example of exogenous anabolic steroid. Inhaled salbutamol – max. 1600 mcg over 24 hours But not to exceed 800 mcg over any 12 hours • LGD-4033 and RAD140 were added as further examples 0 12 24 of SARMs. Any 12 hour period: max. 800 mcg PEPTIDE HORMONES, GROWTH S2 FACTORS, RELATED SUBSTANCES 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg AND MIMETICS • For clarity and accuracy Section S2 was reorganized. • Tulobuterol was added as an example. • ARA290 was removed as an example in this section • The statement on the urinary thresholds was improved. because current literature suggests it does not meet inclusion criteria. • Deslorelin, goserelin, nafarelin and triptorelin were HORMONE AND METABOLIC added as examples of 2.1. S4 MODULATORS • Growth Hormone fragments were included in 2.3 with • Clomifene is now stated by its INN. AOD-9604 and hGH 176-191 added as examples; CJC-1293 was added as example of GHRH and • In the absence of an INN, the IUPAC name of GW1516, tabimorelin as a further example of GH secretagogue. -

PE2572 Puberty Blockers
Puberty Blockers What are puberty Puberty blockers are medicines that block puberty-related hormones that blockers? make your body go through puberty. Starting puberty blockers is a decision that is different for everyone. To make the most informed decision, this handout is meant to help you understand: • What is puberty? • What do puberty blockers do? • What are the changes that will happen to my body? • What are the benefits, risks and costs involved? We will work with you to support the decision that is best for you. You can view a video about puberty blockers at seattlechildrens.org/gender. How does puberty Puberty is the process the body goes through to become capable of begin? making a baby (reproduction), as well as reach adult size and brain development. Puberty starts when your brain tells your pituitary gland to start releasing puberty-related hormones. This happens at different ages for different people. During this time, your body starts to increase the amount of certain puberty-related hormones (Luteinizing Hormone (LH) and Follicle- Stimulating Hormone (FSH). This causes your testicles to start producing testosterone or your ovaries start producing estrogen. These hormones do not cause acne, pubic or armpit hair – those are caused by other hormones. Body changes in • Testicle growth (this improves the body’s ability to make testosterone) people with testicles • Penis growth (without puberty • Pubic hair blockers) • Increased acne, increased armpit and facial hair • Rapid growth (growth spurt) • Voice changes (deepens) Body changes in • Breast changes people with ovaries • Changes in body shape, including fuller hips (without puberty • Menstrual periods start (usually more than 2 years after breast changes blockers) begin) 1 of 6 To Learn More Free Interpreter Services • Adolescent Medicine • In the hospital, ask your nurse. -

Antiprogestins, a New Form of Endocrine Therapy for Human Breast Cancer1
[CANCER RESEARCH 49, 2851-2856, June 1, 1989] Antiprogestins, a New Form of Endocrine Therapy for Human Breast Cancer1 Jan G. M. Klijn,2Frank H. de Jong, Ger H. Bakker, Steven W. J. Lamberts, Cees J. Rodenburg, and Jana Alexieva-Figusch Department of Medical Oncology (Division of Endocrine Oncology) [J. G. M. K., G. H. B., C. J. K., J. A-F.J, Dr. Daniel den Hoed Cancer Center, and Department of Endocrinology ¡F.H. d. J., S. W. ]. L.J, Erasmus University, Rotterdam, The Netherlands ABSTRACT especially pronounced effects on the endometrium, decidua, ovaries, and hypothalamo-pituitary-adrenal axis. With regard The antitumor, endocrine, hematological, biochemical, and side effects of chronic second-line treatment with the antiprogestin mifepristone (RU to clinical practice, the drug has currently been used as a contraceptive agent or abortifacient as a result of its antipro 486) were investigated in 11 postmenopausal patients with metastatic breast cancer. We observed one objective response, 6 instances of short- gestational properties (2, 22-24). Based on its antiglucocorti term stable disease, and 4 instances of progressive disease. Mean plasma coid properties, this drug has been used or has been proposed concentrations of adrenocorticotropic hormone (/' < 0.05), cortisol (/' < for treatment of conditions related to excess corticosteroid 0.001), androstenedione (/' < 0.01), and estradici (P < 0.002) increased production such as Cushing's syndrome (19, 25-27) and for significantly during treatment accompanied by a slight decrease of sex treatment of lymphomas (24) and glaucoma (28); because of its hormone binding globulin levels, while basal and stimulated gonadotropi effects on the immune system, the drug has been suggested to levels did not change significantly. -
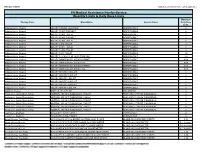
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10%