PE2572 Puberty Blockers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Costs for Treating Central Precocious Puberty During the First Year with Monthly Leuprolide Acetate Injectable and Histrelin Acetate Implant
A Comparison Of The Costs For Treating Central Precocious Puberty During The First Year With Monthly Leuprolide Acetate Injectable And Histrelin Acetate Implant B F Banahan III1, Mayo K2, Summers KH2 1 Center for Pharmaceutical Marketing and Management and Department of Pharmacy Administration, University of Mississippi, University, MS 2 Health Outcomes & PharmacoEconomics, Endo Pharmaceuticals, Inc., Chadds Ford, PA Estimated Annual Cost of Treatment if all PTs Treated With . BACKGROUND METHODS MEDICAID MODEL Lupron SUPPRELIN LA Product costs $ 1,770,201 $ 1,515,188 Two retrospective cohort studies were conducted using datasets derived from the Office visit costs $ 79,896 $ 14,000 Puberty results when secretion of gonadotropin releasing hormone (GnRH) is initiated 100% compliance with Lupron treatment 1 Implant procedures $ 91,400 and the hypothalamic-pituitary-gonadal axis is activated. During puberty, the brain Thomas Reuter’s MarketScan© Multi-State Medicaid Database (2003-2007) and the (All patients receive 13+ treatments in year) MarketScan© Commercial Database (2005-2009). A probabilistic patient flow model Lab/xray for monitoring $ 53,900 $ 37,500 produces GnRH through a complex process. GnRH causes increases in other TOTAL COST TO PAYER $ 1,903,997 $ 1,658,088 hormones like luteinizing hormone (LH) and follicle stimulating hormone (FSH). It is was developed using estimates for treatment patterns and costs for products, office Normal Compliance with Lupron: Lupron SUPPRELIN LA these hormones that cause the ovaries to produce estrogen and the testicles to visits, and monitoring therapy. Patients with < 13 treatments per year Product costs $ 1,572,921 $ 1,515,188 Quality of Care ofCare Quality % of TXd PTs: 53% Aver. -

Hormones and Breeding
IN-DEPTH: REPRODUCTIVE ENDOCRINOLOGY Hormones and Breeding Carlos R.F. Pinto, MedVet, PhD, Diplomate ACT Author’s address: Theriogenology and Reproductive Medicine, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210; e-mail: [email protected]. © 2013 AAEP. 1. Introduction affected by PGF treatment to induce estrus. In The administration of hormones to mares during other words, once luteolysis takes place, whether breeding management is an essential tool for equine induced by PGF treatment or occurring naturally, practitioners. Proper and timely administration of the events that follow (estrus behavior, ovulation specific hormones to broodmares may be targeted to and fertility) are essentially similar or minimally prevent reproductive disorders, to serve as an aid to affected (eg, decreased signs of behavioral estrus). treating reproductive disorders or hormonal imbal- Duration of diestrus and interovulatory intervals ances, and to optimize reproductive efficiency, for are shortened after PGF administration.1 The example, through induction of estrus or ovulation. equine corpus luteum (CL) is responsive to PGF These hormones, when administered exogenously, luteolytic effects any day after ovulation; however, act to control the duration and onset of the different only CL Ͼ5 days are responsive to one bolus injec- stages of the estrous cycle, specifically by affecting tion of PGF.2,3 Luteolysis or antiluteogenesis can duration of luteal function, hastening ovulation es- be reliably achieved in CL Ͻ5 days only if multiple pecially for timed artificial insemination and stimu- PGF treatments are administered. For that rea- lating myometrial activity in mares susceptible to or son, it became a widespread practice to administer showing delayed uterine clearance. -

Puberty Suppression Treatment for Patients with Gender Dysphoria
GENder Education and Care Interdisciplinary Support (GENECIS) Puberty Suppression Treatment for Patients with Gender Dysphoria Patient Information and Informed Parental Consent and Assent for Minors Before considering to give treatment to your child to suppress puberty (put puberty "on hold” with “puberty blockers”), you need to be aware of the possible benefits and risks. After your questions or concerns are addressed and you have decided to proceed with puberty suppression for your child, you will need to initial the statements of this form as well as sign the consent form. If there is more than one parent/legal guardian, both will have to sign. Your child will also need to assent this form. What are the benefits of suppressing puberty in adolescents with gender dysphoria? The Endocrine Society recommends suppression of puberty (put puberty "on hold” with “puberty blockers”), for children that have the diagnosis of gender dysphoria as well as other specific criteria listed in the section below. This recommendation was done by experts in treating youth with gender dysphoria, based on the premise that this may: allow for a smooth social transition to the gender role that is congruent with their gender identity; test persistence of the affirmed gender after living a “real-life experience” and before receiving irreversible hormonal or surgical treatment; and diminish the psychological trauma and risk of suicide induced by the physical changes of puberty. This may also avoid the need for surgery and other expensive treatments that are required to reverse the physical effects of puberty (i.e. mastectomies, tracheal and facial shaving, and electrolysis). -

Farr Handouts
4/14/2021 Recent Pharmacological & Faculty Therapeutic Developments in Women’s Health Glen E. Farr, PharmD Professor Emeritus of Clinical Satellite Conference and Live Webcast Pharmacy and Translational Science April 16, 2021 University of Tennessee 9:00 – 12:00 p.m. Central Time College of Pharmacy [email protected] Produced by the Alabama Department of Public Health Video Communications and Distance Learning Division Educational Objectives Still Have • Outline the recent developments, Sugar & guidelines and/or recommendations Spice for pharmacological management of women’s health conditions, with a focus on contraception. • Identify essential information to counsel patients on the therapeutic application of these medications. Edi the Grand Dog’s first visit to our house 1 4/14/2021 2020 Drug Approvals FDA Annual Novel • 53 Novel agents approved Drug Approvals: – Historical previous 10 year average = 40 2011-2020 • 31 Orphan Drugs • 21 “First in Class” 59 53 48 45 46 • 17 Fast-track 39 41 30 • 22 Break-through 27 22 • 30 Priority review 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 • 12 Accelerated approval FDA.gov Faster FDA Drug Approvals: 3 Question Knowledge Good or Bad? Assessment on • Over the last 4 decades, the FDA has loosened its Contraception requirements for approving new drugs, increasingly accepting less data and more surrogate endpoints in clinical trials, and shortening its reviews. Is this good or bad? • From 1995 to 1997, 80.6% of new drugs were approved on the basis of two pivotal trials — but that number dropped to 52.8% from 2015 to 2017. A number of programs were enacted during the study period that led to faster approvals, such as fast track and the breakthrough therapy designation. -

DESCRIPTION Vantas™ (Histrelin Implant) Is a Sterile Non-Biodegradable, Diffusion-Controlled Reservoir Drug Delivery System De
Vantas™ (histrelin implant) PACKAGE INSERT DESCRIPTION Vantas™ (histrelin implant) is a sterile non-biodegradable, diffusion-controlled reservoir drug delivery system designed to deliver histrelin continuously for 12 months upon subcutaneous implantation. The Vantas implant contains 50 mg of histrelin acetate. Histrelin acetate is a synthetic nonapeptide analogue of the naturally occurring gonadotropin releasing hormone (GnRH) or luteinizing hormone releasing hormone (LH-RH). The sterile Vantas implantation device (provided with the implant) is used to insert the implant subcutaneously in the inner aspect of the upper arm. After 12 months, the implant must be removed. At the time the implant is removed, another implant may be inserted to continue therapy. The sterile Vantas ™ implant consists of a 50-mg histrelin acetate drug core inside a non- biodegradable, 3 cm by 3.5 mm cylindrically shaped hydrogel reservoir (Figure A). The drug core also contains the inactive ingredient stearic acid NF. The hydrogel reservoir is a hydrophilic polymer cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. The hydrated implant is packaged in a glass vial containing 2.0 mL of 1.8% NaCl solution. The implant is primed for release of the drug upon insertion. Figure A. Vantas Histrelin Implant diagram (not to scale) Hydrogel Reservoir Drug Formulation Histrelin acetate is chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L- tyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) [C66H86N18O12. (1.7-2.8 moles) CH3COOH, (0.6-7.0 moles) H2O], with the molecular weight of 1443.70 (or 1323.50 as histrelin base. -

Gender Dysphoria in Children and Adolescents: Medical Considerations
4/12/2016 Gender Dysphoria in Children and Adolescents: Medical Considerations Elyse Pine, MD Staff Physician Chase Brexton Health Care April 30, 2016 I have no relevant financial relationships with commercial interests I WILL be discussing off‐label uses of medications Medical Interventions 1 4/12/2016 Doing Nothing Can be Harmful! Physical Interventions for Adolescents • 1. Fully reversible interventions. – GnRH agonists “puberty blockers” – Spironolactone to decrease testosterone – Oral contraceptives or Progestin to suppress menses • 2. Partially reversible interventions. – Hormone therapy • 3. Irreversible interventions. – Surgical procedures. Typical Puberty • Female puberty ‐ onset (breast bud) age 8 to age 13 – menarche < 5 years from breast bud • Male puberty ‐ onset (testicular enlargement) > age 9, to onset by age 14 • Pace of puberty – Typically 2 years from breast bud to first period – Typically 2 years from testicular enlargement to growth spurt, voice changes, facial hair 2 4/12/2016 Female Pubertal Development Male Pubertal Development “Side Effects” of Puberty • Masculinizing changes of puberty – Deepening of voice – Adam’s apple – Facial and body hair – Skeletal changes of face • Feminizing changes of puberty – Breast growth – Change in body shape‐ hips/thighs – Menstrual cycles (reversible) 3 4/12/2016 The Dutch Protocol • GnRH agonists at age 12, cross sex hormone treatment at 16. • The first 70 Dutch candidates treated with GnRH analogs between 2000 and 2008 showed improved psychological functioning. • None opted to discontinue pubertal suppression and all eventually began cross‐sex hormone treatment. • More recently, the Amsterdam group found that adolescents with GID who underwent pubertal suppression had improved behavioral, emotional, and depressive symptoms with psychometric testing. -

Transgender Health: Helping Your Trans Patient to Live Their Life More
TRANSGENDER HEALTH Helping your trans patient to live their life more easily. Gender affirming services Putting you in charge of your gender journey A patient's perspective Trans life is sacrificing everything for peace of mind @VanessaS2hart Menu 2 INTRODUCTION 5 About this reference guide 5 GenderGP 6 Forward 7 Acknowledgement 8 Understanding gender identity 9 The trans umbrella 9 Gender at a glance 11 Sexuality 11 Transitioning 12 Prevalence 16 Primary healthcare team 17 Barriers to care 20 Training 20 Fear 21 Research 22 The importance of affirmation 23 Real life experience (RLE) 23 Patient groups 25 Adults 25 Older people 26 Towards the end of life 27 Children 28 Adolescents 29 Learning disabilities 32 Autistic spectrum 32 Transgender care: specialist or generalist? 34 The Royal College of General Practitioners (RCGP) 36 Creating a trans-friendly practice 37 First presentation 39 Considerations in primary care 42 General care 42 Gender dysphoria 43 Initiating treatment 44 Continuing care 45 Common scenarios 46 NHS Gender Markers 46 The process for changing gender marker 49 Gender Recognition Certificates (GRC) 49 Screening and diagnosis 50 Referral to a Gender Identity Clinic 51 Waiting times 53 3 Private Care 53 Going abroad for treatment 55 Legal requirements when treating trans patients 55 Conversion therapy 56 Coercion 57 Assessment and diagnosis 58 Criteria for treatment 60 Counselling and support 61 Medical management 62 Hormone therapy 62 Self-medication 64 Unlicensed medicines 64 Side effects 65 Informed consent 65 An important -

Gender-Affirming Healthcare
Closer look at topical conditions How to treat You can earn 1 credit by completing the ELearning assessment for this article 1 CR at nzdoctor.co.nz Gender-affirming healthcare This article covers the diverse aspects of providing gender-affirming healthcare for Aotearoa’s transgender and non-binary people, including the use of puberty blockers and hormone therapy, as well as the general principles behind this rapidly evolving area of medicine. It was written by Cathy Stephenson, Alex Ker and Rachel Johnson, and was peer-reviewed by the Professional Association for Transgender Health Aotearoa board A note on language: throughout this article, we use the um- background and access to resources. For some people, tran- Cathy Do you need to read this article? brella term “transgender and non-binary” to describe people Stephenson sitioning is seen as a collective process involving the person’s whose gender is different from the sex they were assigned at (she/her) is a whānau. birth. Gender diversity is recognised, expressed and celebrated GP at Mauri People may face various barriers to transitioning, such Try this quiz in many indigenous cultures, including te ao Māori and across Ora, Victoria as lack of family support or financial resources. Because 1. Transgender and non-binary patients have the the Pacific. These cultures have their own understandings and University of gender-affirming services in Aotearoa are not funded con- same routine health needs as every other patient. Wellington, True/False histories of gender diversity. People may use culturally specif- and a member sistently across DHBs, some people’s ability to transition ic language, such as takatāpui and fa’afafine, to describe their of Capital and are also limited by “postcode lottery”, or their geographic 2. -
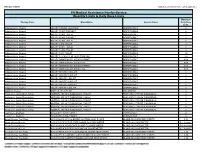
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10% -

DEAR PHYSICIAN: This Letter Is Being Provided As a Sample to Help You with Your Payor Interactions Concerning Reimbursement
DEAR PHYSICIAN: This letter is being provided as a sample to help you with your payor interactions concerning reimbursement for the administration of SUPPRELIN® LA (histrelin acetate) subcutaneous implant. Use of this document does not guarantee coverage or reimbursement. As a healthcare professional, you are solely responsible for providing accurate information to third-party payors. If there is any information in this document that does not accurately reflect your practices, it should be modified to appropriately represent your particular circumstances. INDICATION • SUPPRELIN® LA (histrelin acetate) subcutaneous implant is indicated for the treatment of children with central precocious puberty (CPP). • Children with CPP (neurogenic or idiopathic) have an early onset of secondary sexual characteristics (earlier than 8 years of age in females and 9 years of age in males). They also show a significantly advanced bone age that can result in diminished adult height attainment. • Prior to initiation of treatment, a clinical diagnosis of CPP should be confirmed by measurement of blood concentrations of total sex steroids, luteinizing hormone (LH) and follicle stimulating hormone (FSH) following stimulation with a GnRH analog, and assessment of bone age versus chronological age. Baseline evaluations should include height and weight measurements, diagnostic imaging of the brain (to rule out intracranial tumor), pelvic/testicular/adrenal ultrasound (to rule out steroid secreting tumors), human chorionic gonadotropin levels (to rule out a chorionic gonadotropin secreting tumor), and adrenal steroids to exclude congenital adrenal hyperplasia. IMPORTANT SAFETY INFORMATION ABOUT SUPPRELIN® LA • SUPPRELIN® LA is contraindicated in patients who are hypersensitive to gonadotropin releasing hormone (GnRH) or GnRH agonist analogs and in females who are or may become pregnant while receiving the drug. -

Information About Puberty Blockers
Information About Puberty Blockers Before considering a medication for yourself/your child to put puberty “on hold,” there are several things you need to know. There are possible advantages, disadvantages, and risks with puberty blockers. It's important that you understand all of this information before any medication treatment begins. Please read the following carefully and ask any questions. We want you to be very comfortable and sure of what pubertal blockers offer. Here are some additional resources: http://www.impactprogram.org/wp-content/uploads/2014/12/Kuper-2014-Puberty-Blockers-Clinic al-Research-Review.pdf https://www.ted.com/speakers/norman_spack http://www.pbs.org/wgbh/pages/frontline/growing/ Recommendations for Hormone Therapy/Puberty Suppressing Hormones (based on UCSF, WPATH, and the Pediatric Endocrine Society) In order for adolescents to be candidates for puberty suppressing therapies, QueerDoc looks for: 1. A long-lasting and intense pattern of gender nonconformity or gender dysphoria (whether suppressed or expressed); 2. Gender dysphoria emerged or worsened with the onset of puberty; 3. Any co-existing psychological, medical, or social problems that could interfere with treatment (for example by compromising treatment adherence) have been addressed, such that the adolescent’s situation and functioning are stable enough to start treatment; 4. The adolescent has given informed consent and, particularly for minors, the parents/ guardians have consented to the treatment and are involved in supporting the adolescent throughout the treatment process. What are the different medications that can help to stop the physical changes of puberty? The main way that the physical changes of puberty can be put on hold is by blocking the signal from the brain to the organs that make the hormones of puberty. -

Erleada (Apalutamide)
Market Applicability Market GA KY MD NJ NY WA Applicable X X X X X X Erleada (apalutamide) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit Medications Quantity Limit Erleada (apalutamide) May be subject to quantity limit APPROVAL CRITERIA Requests for Erleada (apalutamide) may be approved if the following criteria are met: I. Individual is diagnosed with one of the following: A. Individual has a diagnosis of non-metastatic castration-resistant* prostate cancer (nmCRPC); OR B. Individual has a diagnosis of metastatic castration-sensitive prostate cancer (mCSPC) AND II. One of the following: A. Individual is concomitantly receiving a gonadotropin-releasing hormone (GnRH) analog (e.g. Lupron (leuprolide, Zoladex (goserelin), Trelstar (triptorelin), Vantas (histrelin), Firmagon (degarelix); OR B. Individual has had a bilateral orchiectomy. *Castration-resistant refers to either surgical or medically induced methods. Medically induced methods include luteinizing hormone-releasing hormone (LHRH) agonists (such as leuprolide, goserelin) or LHRH antagonists (such as degarelix). Key References: 1. Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.: 2020. URL: http://www.clinicalpharmacology.com. Updated periodically. 2. DailyMed. Package inserts. U.S. National Library of Medicine, National Institutes of Health website. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed: April 19, 2020. PAGE 1 of 2 09/11/2020 CRX-ALL-0595-20 New Program Date 04/09/2018 This policy does not apply to health plans or member categories that do not have pharmacy benefits, nor does it apply to Medicare. Note that market specific restrictions or transition-of-care benefit limitations may apply. Market Applicability Market GA KY MD NJ NY WA Applicable X X X X X X 3.