Plasma Bile Acid Profile in Patients with and Without Type 2 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review: Microbial Transformations of Human Bile Acids Douglas V
Guzior and Quinn Microbiome (2021) 9:140 https://doi.org/10.1186/s40168-021-01101-1 REVIEW Open Access Review: microbial transformations of human bile acids Douglas V. Guzior1,2 and Robert A. Quinn2* Abstract Bile acids play key roles in gut metabolism, cell signaling, and microbiome composition. While the liver is responsible for the production of primary bile acids, microbes in the gut modify these compounds into myriad forms that greatly increase their diversity and biological function. Since the early 1960s, microbes have been known to transform human bile acids in four distinct ways: deconjugation of the amino acids glycine or taurine, and dehydroxylation, dehydrogenation, and epimerization of the cholesterol core. Alterations in the chemistry of these secondary bile acids have been linked to several diseases, such as cirrhosis, inflammatory bowel disease, and cancer. In addition to the previously known transformations, a recent study has shown that members of our gut microbiota are also able to conjugate amino acids to bile acids, representing a new set of “microbially conjugated bile acids.” This new finding greatly influences the diversity of bile acids in the mammalian gut, but the effects on host physiology and microbial dynamics are mostly unknown. This review focuses on recent discoveries investigating microbial mechanisms of human bile acids and explores the chemical diversity that may exist in bile acid structures in light of the new discovery of microbial conjugations. Keywords: Bile acid, Cholic acid, Conjugation, Microbiome, Metabolism, Microbiology, Gut health, Clostridium scindens, Enterocloster bolteae Introduction the development of healthy or diseased states. For The history of bile example, abnormally high levels of the microbially modi- Bile has been implicated in human health for millennia. -

The Analysis of Bile Acids: Enhancement of Specificity Using an Ion Mobility-Tofms Based Approach
THE ANALYSIS OF BILE ACIDS: ENHANCEMENT OF SPECIFICITY USING AN ION MOBILITY-TOFMS BASED APPROACH Jonathan P Williams1, Martin Palmer1, Jonas Abdel-Khalik2, Yuqin Wang2, Sarah M Stow3, Mark Towers1, Giuseppe Astarita1, James Langridge1 and William J Griffiths2 1 2 3 Waters Corporation, Wilmslow, Manchester UK; College of Medicine, Swansea University UK; Laboratory for Structural Mass Spectrometry, Vanderbilt University, TN, USA Fig.1 shows a schematic of Vion. In brief, the instrument com- MALDI Imaging Ion Mobility MS measurements of the 100 Representative conformations from distance geometry INTRODUCTION prises an IM separation device, a quadrupole and segmented RESULTS isomeric bile acids deoxycholic acid and hyodeoxy-cholic modeling for the bile acids investigated collision cell prior to the TOFMS. Ions are accumulated in the acid DCA 'Steroidomics' is the qualitative and quantitative trap travelling-wave (T-Wave) and periodically released into New and improved methods were sought for the identification, HA quantification, and characterization of bile acids, oxysterols, and other the T-Wave IM where they separate according to their mobil- % study of steroid-type molecules found within the sterols and steroids. The involvement of these molecules in metabolome. Bile acids for example, are ity. neurogenesis and immunity is investigated. classified as acidic sterols that are synthesised The use of IM as an analytical tool to aid direct infusion ESI, DESI and mainly by the liver from cholesterol and aid MALDI shotgun steroidomic-type analysis was investigated. Bile acids 0 digestion and fat solubilisation. The presence of present themselves in biological type samples as complex mixtures. 2.00 2.50 3.00 3.50 4.00 4.50 5.00 multiple isomeric bile acids poses a great Structural information may be obtained using MS/MS but in the absence of a chromatographic step, unambiguous characterisation using MS/MS 100 challenge for steroidomic research. -

Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver–Gut Axis
International Journal of Molecular Sciences Review Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver–Gut Axis Niklas Grüner 1 and Jochen Mattner 1,2,* 1 Mikrobiologisches Institut-Klinische Mikrobiologie, Immunologie und Hygiene, Universitätsklinikum Erlangen and Friedrich-Alexander Universität (FAU) Erlangen-Nürnberg, 91054 Erlangen, Germany; [email protected] 2 Medical Immunology Campus Erlangen, FAU Erlangen-Nürnberg, 91054 Erlangen, Germany * Correspondence: [email protected]; Tel.: +49-9131-852-3640 Abstract: After their synthesis from cholesterol in hepatic tissues, bile acids (BAs) are secreted into the intestinal lumen. Most BAs are subsequently re-absorbed in the terminal ileum and are trans- ported back for recycling to the liver. Some of them, however, reach the colon and change their physicochemical properties upon modification by gut bacteria, and vice versa, BAs also shape the composition and function of the intestinal microbiota. This mutual interplay of both BAs and gut microbiota regulates many physiological processes, including the lipid, carbohydrate and energy metabolism of the host. Emerging evidence also implies an important role of this enterohepatic BA circuit in shaping mucosal colonization resistance as well as local and distant immune responses, tissue physiology and carcinogenesis. Subsequently, disrupted interactions of gut bacteria and BAs are associated with many disorders as diverse as Clostridioides difficile or Salmonella Typhimurium infection, inflammatory bowel disease, type 1 diabetes, asthma, metabolic syndrome, obesity, Parkin- son’s disease, schizophrenia and epilepsy. As we cannot address all of these interesting underlying pathophysiologic mechanisms here, we summarize the current knowledge about the physiologic and pathogenic interplay of local site microbiota and the enterohepatic BA metabolism using a few Citation: Grüner, N.; Mattner, J. -

Applications of Chemical Methodology in Environmental Science, Systems Biology, and Interdisciplinary Chemical Education
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2019 Applications of Chemical Methodology in Environmental Science, Systems Biology, and Interdisciplinary Chemical Education Caleb Michael Gibson University of Tennessee, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Recommended Citation Gibson, Caleb Michael, "Applications of Chemical Methodology in Environmental Science, Systems Biology, and Interdisciplinary Chemical Education. " PhD diss., University of Tennessee, 2019. https://trace.tennessee.edu/utk_graddiss/5400 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Caleb Michael Gibson entitled "Applications of Chemical Methodology in Environmental Science, Systems Biology, and Interdisciplinary Chemical Education." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Chemistry. Shawn Campagna, Major Professor We have read this dissertation and recommend its acceptance: Elizabeth Fozo, MIchael Sepaniak, Ampofo Darko Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) APPLICATIONS OF CHEMICAL METHODOLOGY IN ENVIRONMENTAL SCIENCE, SYSTEMS BIOLOGY, AND INTERDISCIPLINARY CHEMICAL EDUCATION A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Caleb Michael Gibson May 2019 Copyright © 2019 by Caleb Michael Gibson All rights reserved. -

Effect of Exogenous Glucocorticoid on Osmotically Stimulated Antidiuretic
European Journal of Endocrinology (2006) 155 845–848 ISSN 0804-4643 CLINICAL STUDY Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man Volker Ba¨hr1, Norma Franzen1, Wolfgang Oelkers2, Andreas F H Pfeiffer1 and Sven Diederich1,2 1Department of Endocrinology, Diabetes and Nutrition, Charite-Universitatsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany and 2Endokrinologikum Berlin, Centre for Endocrine and Metabolic Diseases, Berlin, Germany (Correspondence should be addressed to V Ba¨hr; Email: [email protected]) Abstract Objective: Glucocorticoids exert tonic suppression of antidiuretic hormone (ADH) secretion. Hypocortisolism in secondary adrenocortical insufficiency can result in a clinical picture similar to the syndrome of inappropriate ADH secretion. On the other hand, in vitro and in vivo results provide evidence for ADH suppression in states of hypercortisolism. To test the hypothesis that ADH suppression is of relevance during glucocorticoid therapy, we investigated the influence of prednisolone on the osmotic stimulation of ADH. Design and methods: Seven healthy men were subjected to water deprivation tests with the measurement of plasma ADH (pADH) and osmolality (posmol) before and after glucocorticoid treatment (5 days 30 mg prednisolone per day). Results: Before glucocorticoid treatment, the volunteers showed a normal test with an adequate increase of pADH (basal 0.54G0.2 to 1.9G0.72 pg/ml (meanGS.D.)) in relation to posmol(basal 283.3G8.5 to 293.7G6 mosmol/kg). After prednisolone intake, pADH was attenuated (!0.4 pg/ml) in spite of an increase of posmol from 289.3G3.6 to 297.0G5.5 mosmol/kg. -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma. -
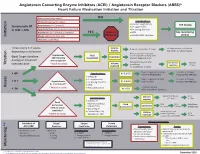
Initiation Titration Assess Monitoring **
Angiotensin Converting Enzyme Inhibitors (ACEI) / Angiotensin Receptor Blockers (ARBS)* Heart Failure Medication Initiation and Titration Bilateral renal artery stenosis NO Moderate/Severe aortic stenosis Considerations: • Baseline cough (ACEi) Hyperkalemia: K+ > 5.2 mmol/L See dosing Symptomatic HF • K+ supplements or LVEF < 40% Renal Dysfunction:Serum creatinine >220 µmol/L • K+ sparing diuretics Hypotension: SBP < 90 mmHg or symptoms YES Refer to • MRA See monitoring Initiation Allergy: angioedema, hives, rash Physician • NSAIDS/COX2 inhibitors section Intolerance: cough (ACEi) Titrate every 1-3 weeks, Volume Reduce/hold diuretic x 2-3 days No improvement, hold/reduce depending on tolerance deplete ACEI/ARB x 1-2 wks & reassess Reassess diuretic dose/other Hypotension Fluid non-essential BP lowering meds Goal: Target dose(see Euvolemic SBP<90mmHg Assessment Consider staggering doses dosing) or maximum with symptoms* Reduce/hold dose of other See Diuretic Reassess Titration * Watch for trends Volume tolerated dose vasodilators algorithm 1-2 wks overload +/- ACEI/ARB x 1-2 weeks Stop K+ supplements, reduce/ Serum K+ in 3-5 days, Considerations: • BP K+ 5.2-5.5 hold MRA (if applicable) Reassess ACEI/ARB dose • Dietary K+ • K+ supplements Stop K+ supplements, MRA. Serum K+ in 2-3 days, • K + Hyperkalemia • K+ sparing diuretics K+ 5.6-6.0 hold ACEI/ARB Reassess ACEI/ARB dose K+ > 5.2 mmol/L* • MRA Assess * Watch for trends • Renal dysfunction Treat hyperkalemia • Scr K+ > 6.0 Refer to MD/NP +/- send to ED Reduce/hold diuretic Scr in Considerations: -

Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax)
Caffeine and Energy Boosting Drugs: Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax) www.ctclearinghouse.org A Library and Resource Center on Alcohol, Tobacco, Other Drugs, Mental Health and Wellness What are energy drinks? you. You wouldn't use Mountain Dew as a sports Energy drinks are beverages like drink. And a drink like Red Bull and vodka is Red Bull, Venom, Adrenaline Rush, more like strong coffee and whisky than 180, ISO Sprint, and Whoopass, anything else. which contain large doses of caffeine and other legal stimulants What happens when energy drinks are like ephedrine, guarana, and ginseng. combined with alcohol? Energy drinks may contain as much as 80 mg. Energy drinks are also used as mixers with of caffeine, the equivalent of a cup of coffee. alcohol. This combination carries a number of Compared to the 37 mg. of caffeine in a dangers: Mountain Dew, or the 23 mg. in a Coca-Cola Classic, that's a big punch. These drinks are • Since energy drinks are stimulants and marketed to people under 30, especially to alcohol is a depressant, the combination of college students, and are widely available both effects may be dangerous. The stimulant on and off campus. effects can mask how intoxicated you are and prevent you from realizing how much Are there short-term dangers to drinking alcohol you have consumed. Fatigue is one energy drinks? of the ways the body normally tells Individual responses to caffeine vary, and someone that they've had enough to drink. these drinks should be treated carefully • The stimulant effect can give the person the because of how powerful they are. -

The Necessity and Effectiveness of Mineralocorticoid Receptor Antagonist in the Treatment of Diabetic Nephropathy
Hypertension Research (2015) 38, 367–374 & 2015 The Japanese Society of Hypertension All rights reserved 0916-9636/15 www.nature.com/hr REVIEW The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy Atsuhisa Sato Diabetes mellitus is a major cause of chronic kidney disease (CKD), and diabetic nephropathy is the most common primary disease necessitating dialysis treatment in the world including Japan. Major guidelines for treatment of hypertension in Japan, the United States and Europe recommend the use of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, which suppress the renin-angiotensin system (RAS), as the antihypertensive drugs of first choice in patients with coexisting diabetes. However, even with the administration of RAS inhibitors, failure to achieve adequate anti-albuminuric, renoprotective effects and a reduction in cardiovascular events has also been reported. Inadequate blockade of aldosterone may be one of the reasons why long-term administration of RAS inhibitors may not be sufficiently effective in patients with diabetic nephropathy. This review focuses on treatment in diabetic nephropathy and discusses the significance of aldosterone blockade. In pre-nephropathy without overt nephropathy, a mineralocorticoid receptor antagonist can be used to enhance the blood pressure-lowering effects of RAS inhibitors, improve insulin resistance and prevent clinical progression of nephropathy. In CKD categories A2 and A3, the addition of a mineralocorticoid receptor antagonist to an RAS inhibitor can help to maintain ‘long-term’ antiproteinuric and anti-albuminuric effects. However, in category G3a and higher, sufficient attention must be paid to hyperkalemia. Mineralocorticoid receptor antagonists are not currently recommended as standard treatment in diabetic nephropathy. -

Peroxisome Proliferator-Activated Receptor Induces Hepatic
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 278, No. 35, Issue of August 29, pp. 32852–32860, 2003 © 2003 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Peroxisome Proliferator-activated Receptor ␣ Induces Hepatic Expression of the Human Bile Acid Glucuronidating UDP-glucuronosyltransferase 2B4 Enzyme* Received for publication, May 22, 2003 Published, JBC Papers in Press, June 16, 2003, DOI 10.1074/jbc.M305361200 Olivier Barbier‡, Daniel Duran-Sandoval‡, Ine´s Pineda-Torra‡, Vladimir Kosykh§, Jean-Charles Fruchart‡, and Bart Staels‡¶ From the ‡Unite´de Recherche 545, Institut National de la Sante´et de la Recherche Me´dicale (INSERM), De´partement d’Athe´roscle´rose, Institut Pasteur de Lille and the Faculte´de Pharmacie, Universite´de Lille II, 59019 Lille, France and §Institute of Experimental Cardiology, Russian Cardiology Complex, Moscow 121552, Russia Glucuronidation, a major metabolic pathway for a pollutants (1). This reaction consists in the transfer of the large variety of endobiotics and xenobiotics, is cata- glucuronosyl group from UDP-glucuronic acid to the acceptor lyzed by enzymes belonging to the UDP-glucuronosyl- molecule (1). The addition of the glucuronosyl group on a com- transferase (UGT) family. Among UGT enzymes, pound results in a more water-soluble molecule, which can be UGT2B4 conjugates a large variety of endogenous and excreted into bile or urine. Glucuronidation is catalyzed by Downloaded from exogenous molecules and is considered to be the major enzymes belonging to the UDP-glucuronosyltransferase (UGT) bile acid conjugating UGT enzyme in human liver. In the family, and based on primary structure homology, UGT pro- present study, we identify UGT2B4 as a novel target teins have been divided into two major subfamilies, UGT1A gene of the nuclear receptor peroxisome proliferator- and UGT2B (2). -

Optimal Diuretic Strategies in Heart Failure
517 Review Article on Heart Failure Update and Advances in 2021 Page 1 of 8 Optimal diuretic strategies in heart failure Sarabjeet S. Suri, Salpy V. Pamboukian Division of Cardiovascular Diseases, University of Alabama at Birmingham, Birmingham, AL, USA Contributions: (I) Conception and design: SS Suri; (II) Administrative support: SV Pamboukian; (III) Provision of study materials or patients: Both authors; (IV) Collection and assembly of data: Both authors; (V) Data analysis and interpretation: Both authors; (VI) Manuscript writing: Both authors; (VII) Final approval of manuscript: Both authors. Correspondence to: Dr. Sarabjeet S. Suri, MD. University of Alabama at Birmingham, 1900 University Blvd, THT 321, Birmingham, AL 35223, USA. Email: [email protected]. Abstract: Heart failure (HF) is one of the major causes of morbidity and mortality in the world. According to a 2019 American Heart Association report, about 6.2 million American adults had HF between 2013 and 2016, being responsible for almost 1 million admissions. As the population ages, the prevalence of HF is anticipated to increase, with 8 million Americans projected to have HF by 2030, posing a significant public health and financial burden. Acute decompensated HF (ADHF) is a syndrome characterized by volume overload and inadequate cardiac output associated with symptoms including some combination of exertional shortness of breath, orthopnea, paroxysmal nocturnal dyspnea (PND), fatigue, tissue congestion (e.g., peripheral edema) and decreased mentation. The pathology is characterized by hemodynamic abnormalities that result in autonomic imbalance with an increase in sympathetic activity, withdrawal of vagal activity and neurohormonal activation (NA) resulting in increased plasma volume in the setting of decreased sodium excretion, increased water retention and in turn an elevation of filling pressures. -

Combination Therapy
Combination Antihypertensive Therapy: When to use it Diabetes George L. Bakris, MD, F.A.S.N., F.A.S.H. Professor of Medicine Director, ASH Comprehensive Hypertension Center The University of Chicago Medicine Development of Antihypertensive Therapies ? More Effective but As effective and As effective and even effective for poorly tolerated better tolerated better tolerated SBP 1940s 1950 1957 1960s 1970s 1980s 1990s 2001- 2009 ARBs Direct Direct ACE inhibitors Renin vasodilators inhibitors α-blockers Peripheral Thiazides ETa sympatholytics diuretics Blockers Central ααα 2 Calcium Ganglion agonists blockers antagonists- VPIs Calcium DHPs Others Veratrum antagonists- alkaloids non DHPs βββ-blockers Evolution of Fixed Dose Combination Antihypertensive Therapies 1960s 1970s 1980s 1990s 2000- present Combination CCBs+ ARBs Diuretics RAS Blockers ARB + Aldactazide, with CCBs chlorthalidone Dyazide, Maxzide, DRIs +ARBs Guanabenz SerApAs RAS Blockers (Lotrel) DRIs+ CCBs (reserpine, with diuretics hydralazine, Beta blocker +diuretics TRIPLE Combos HCTZ) (CCB+RAS Blocker + diuretic) Rationale for Fixed-Dose Combination Therapy: Background • Traditional antihypertensive therapy yields goal BP in <60% of treated hypertensive patients1-3 • Switching from one monotherapy to another is effective in only about 50% of patients1 • Most patients will require at least two drugs to attain goal BP (<140/90 mm Hg, or <130/80 mm Hg for patients with diabetes or chronic renal disease)4-6 BP = blood pressure 1. Materson BJ et al. J Hum Hypertens. 1995;9(10):791-796. 2. Messerli FH. J Hum Hypertens. 1992;6 Suppl. 2:S19-S21. 3. Ram CV. J Clin Hypertens (Greenwich). 2004;6(10):569-577. 4. Chobanian AV, et al. JAMA.