Clinical Pharmacology in Diuretic Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DIURETICS Diuretics Are Drugs That Promote the Output of Urine Excreted by the Kidneys
DIURETICS Diuretics are drugs that promote the output of urine excreted by the Kidneys. The primary action of most diuretics is the direct inhibition of Na+ transport at one or more of the four major anatomical sites along the nephron, where Na+ reabsorption takes place. The increased excretion of water and electrolytes by the kidneys is dependent on three different processes viz., glomerular filtration, tubular reabsorption (active and passive) and tubular secretion. Diuretics are very effective in the treatment of Cardiac oedema, specifically the one related with congestive heart failure. They are employed extensively in various types of disorders, for example, nephritic syndrome, diabetes insipidus, nutritional oedema, cirrhosis of the liver, hypertension, oedema of pregnancy and also to lower intraocular and cerebrospinal fluid pressure. Therapeutic Uses of Diuretics i) Congestive Heart Failure: The choice of the diuretic would depend on the severity of the disorder. In an emergency like acute pulmonary oedema, intravenous Furosemide or Sodium ethacrynate may be given. In less severe cases. Hydrochlorothiazide or Chlorthalidone may be used. Potassium-sparing diuretics like Spironolactone or Triamterene may be added to thiazide therapy. ii) Essential hypertension: The thiazides usually sever as primary antihypertensive agents. They may be used as sole agents in patients with mild hypertension or combined with other antihypertensives in more severe cases. iii) Hepatic cirrhosis: Potassium-sparing diuretics like Spironolactone may be employed. If Spironolactone alone fails, then a thiazide diuretic can be added cautiously. Furosemide or Ethacrymnic acid may have to be used if the oedema is regractory, together with spironolactone to lessen potassium loss. Serum potassium levels should be monitored periodically. -

Effect of Exogenous Glucocorticoid on Osmotically Stimulated Antidiuretic
European Journal of Endocrinology (2006) 155 845–848 ISSN 0804-4643 CLINICAL STUDY Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man Volker Ba¨hr1, Norma Franzen1, Wolfgang Oelkers2, Andreas F H Pfeiffer1 and Sven Diederich1,2 1Department of Endocrinology, Diabetes and Nutrition, Charite-Universitatsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany and 2Endokrinologikum Berlin, Centre for Endocrine and Metabolic Diseases, Berlin, Germany (Correspondence should be addressed to V Ba¨hr; Email: [email protected]) Abstract Objective: Glucocorticoids exert tonic suppression of antidiuretic hormone (ADH) secretion. Hypocortisolism in secondary adrenocortical insufficiency can result in a clinical picture similar to the syndrome of inappropriate ADH secretion. On the other hand, in vitro and in vivo results provide evidence for ADH suppression in states of hypercortisolism. To test the hypothesis that ADH suppression is of relevance during glucocorticoid therapy, we investigated the influence of prednisolone on the osmotic stimulation of ADH. Design and methods: Seven healthy men were subjected to water deprivation tests with the measurement of plasma ADH (pADH) and osmolality (posmol) before and after glucocorticoid treatment (5 days 30 mg prednisolone per day). Results: Before glucocorticoid treatment, the volunteers showed a normal test with an adequate increase of pADH (basal 0.54G0.2 to 1.9G0.72 pg/ml (meanGS.D.)) in relation to posmol(basal 283.3G8.5 to 293.7G6 mosmol/kg). After prednisolone intake, pADH was attenuated (!0.4 pg/ml) in spite of an increase of posmol from 289.3G3.6 to 297.0G5.5 mosmol/kg. -

Nephropharmacology for the Clinician
Nephropharmacology for the Clinician Clinical Pharmacology in Diuretic Use David H. Ellison CJASN 14: 1248–1257, 2019. doi: https://doi.org/10.2215/CJN.09630818 Diuretics are among the most commonly prescribed Gastrointestinal Absorption of Diuretics drugs and, although effective, they are often used to The normal metabolism of loop diuretics is shown in treat patients at substantial risk for complications, Figure 2A. Furosemide, bumetanide, and torsemide are Departments of making it especially important to understand and absorbed relatively quickly after oral administration Medicine and appreciate their pharmacokinetics and pharmacody- (see Figure 2B), reaching peak concentrations within Physiology and – Pharmacology, namics (see recent review by Keller and Hann [1]). 0.5 2 hours (3,4); when administered intravenously, Oregon Health & Although the available diuretic drugs possess distinc- their effects are nearly instantaneous. The oral bioavail- Science University, tive pharmacokinetic and pharmacodynamic proper- ability of bumetanide and torsemide typically exceeds Portland, Oregon; and ties that affect both response and potential for adverse 80%, whereas that of furosemide is substantially lower, Renal Section, at approximately 50% (see Table 2) (5). Although the t Veterans Affairs effects, many clinicians use them in a stereotyped 1/2 Portland Health Care manner, reducing effectiveness and potentially in- of furosemide is short, its duration of action is longer System, Portland, creasing side effects (common diuretic side effects are when administered orally, as its gastrointestinal Oregon t listed in Table 1). Diuretics have many uses, but this absorption may be slower than its elimination 1/2. review will focus on diuretics to treat extracellular This is a phenomenon called “absorption-limited Correspondence: fluid (ECF) volume expansion and edema; the reader is kinetics” (3) and may explain the mnemonic that Dr. -

Chlorothiazide Versus Metolazone in Hospitalized Patients with Heart
Chlorothiazide Versus Metolazone in Hospitalized Patients with Heart Failure Receiving Loop Diruetics Barry Nicholson, PharmD; Halley Gibson, PharmD, BCPS Lahey Hospital & Medical Center, Burlington, MA Background Baseline Characteristics Secondary Outcomes Results • Heart failure (HF) is the primary diagnosis in > 1 million hospitalizations annually Characteristic Metolazone Chlorothiazide p-value • Loop diuretics are first-line treatment for edema for most patients with HF, and Secondary Outcome Metolazone Chlorothiazide p-value (n=62) (n=59) thiazide diuretics may be used as an adjuvant option for additional diuresis1 (n=62) (n=59) • Previous trials have shown that oral metolazone is non-inferior to intravenous chlorothiazide with regards to safety and efficacy2,3 Age (years) ± SD 75.6 ± 12.1 74.8 ± 12.1 0.55 Hypokalemia within 24 hours 13 (21) 14 (23.7) 0.89 • There is currently no institution-specific policy to guide thiazide diuretic post-thiazide, n (%) Male, n (%) 40 (64.5) 34 (56.7) 0.55 selection in patients with heart failure at Lahey Hospital & Medical Center Hypokalemia within 72 hours 10 (16.1) 5 (8.5) 0.32 post-thiazide, n (%) Objective Weight (kg) ± SD 92 ± 32.8 95.5 ± 31.1 0.54 To evaluate the safety and efficacy of chlorothiazide versus metolazone for Hypomagnesemia within 24 hours 5 (8.1) 7 (11.9) 0.69 Non-ICU, n (%) 48 (77.4) 39 (65) 0.24 augmented diuresis in hospitalized heart failure patients receiving loop diuretics post-thiazide, n (%) Endpoints Progressive Care Unit, n (%) 12 (19.4) 3 (5.1) 0.07 Hypomagnesemia within 72 hours 9 (14.5) 6 (10.2) 0.66 post-thiazide, n (%) Primary Endpoint Change in net urine output (UOP) pre and post-initiation Intensive Care Unit, n (%) 2 (3.2) 17 (28.8) <0.01* of either study thiazide diuretic at 24 and 72 hours Length of Stay, days ± SD 14.7 ± 6.6 17 ± 11.8 0.2 Serum creatinine, mg/dL ± SD 2.2 ± 1.2 2.2 ± 1.1 0.91 Secondary Endpoints - Incidence of the following at 24 and 72 hours: # patients needing additional 27 (43.5) 37 (62.7) 0.05 1. -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma. -

MELT-HF Metolazone As Early Add on Therapy for Acute Decompensated
MELT-HF MEtolazone as Early add on Therapy for acute decompensated Heart Failure. A single center pilot study. Muhammad A. Chaudhry, MD MELT-HF Protocol [Amendment 5.0; 17-Jan-2017] Page 1 of 10 INTRODUCTION Background Heart failure is a major source of morbidity, mortality and growing public health cost. In US, the number of congestive heart failure patients is more than 4 million with more than 550,000 new annually reported cases. The annual cost of heart failure management exceeds 35 billion dollars per year. The heart failure readmissions and average length of hospital stay cost approximately $11,000 per patient. Loop diuretics are used alone in the majority of cases to promote diuresis. An association of increased creatinine and increased risk of renal dysfunction, the cardiorenal syndrome, in the face of high dose loop diuretics has raised questions regarding the safety and toxicity of high dose loop diuretics. While the dose of diuretics is ubiquitous, little data exists to guide their use and many clinicians are uncertain as to when and how to initiate and limit therapy. In many cases, a “stepped approach” with oral loop diuretics advancing to intravenous and finally combination high dose diuretics is employed. PREVIOUS TRIALS DOSE( Diuretic strategies in patients with ADHF) Trial Prospective, randomized double blinded trial of 308 patients with ADHF. Showed that high dose diuretics (2.5 times the outpatient daily dose) were associated with improved urine output at 72 hours (3575± 2635 in low dose group vs. 4899±3479 in high dose group). There was no significant difference between the high and low dose groups in mean creatinine change (0.08±0.3 mg per deciliter in high-dose group as compared to 0.04±0.3 mg per deciliter in low-dose group, P=0.21. -

Diuretic Renal Scintigraphy in Patients with Sulfonamide Allergies: Possible Alternative Use of Ethacrynic Acid
J of Nuclear Medicine Technology, first published online October 15, 2015 as doi:10.2967/jnmt.115.161331 Diuretic Renal Scintigraphy in Patients with Sulfonamide Allergies: Possible Alternative Use of Ethacrynic Acid. Ba D. Nguyen, M.D. Michael C. Roarke, M.D. Jason Robert Young*, M.D. Ming Yang, M.D. Howard H. Osborn R.N. Department of Radiology, Mayo Clinic, Scottsdale, AZ (attributed to work) *Department of Radiology, Maricopa Integrated Health System, Phoenix, AZ Correspondence and first author: Ba D Nguyen, M.D., Department of Radiology, Mayo Clinic, 13400 E. Shea Blvd., Scottsdale, AZ 85259. Telephone: 480 3016865, Fax: 480 3014303, Email: [email protected] Word Count: 999 Financial Support: None Disclosures: Nothing to disclose Short running title: Ethacrynic Acid for Renal Scintigraphy Diuretic Renal Scintigraphy in Patients with Sulfonamide Allergies: Possible Alternative Use of Ethacrynic Acid. Abstract Furosemide may trigger life threatening sulfonamide cross-hypersensitivity reactions, posing an imaging decision dilemma for patients who need diuretic renal scintigraphy. Methods: The authors present the experience using ethacrynic acid as a non-sulfonamide alternative diuretic in five patients with discussion of diuretic molecular structure, potential side effects, protocol development and imaging results. Results: Diuretic renal scintigraphy using ethacrynic acid provided useful information about the obstructive syndrome status in all cases with no adverse clinical impact. Conclusion: Ethacrynic acid is a potential alternative to furosemide for patients with severe sulfonamide reactions. Key words: Ethacrynic Acid, Diuretic Renal Scintigraphy, Sulfonamide allergy. The objective of this essay is to raise awareness of ethacrynic acid as a possible alternative to furosemide for provocative diuretic renal scintigraphy. -

Azosemide Is More Potent Than Bumetanide and Various Other Loop
www.nature.com/scientificreports OPEN Azosemide is more potent than bumetanide and various other loop diuretics to inhibit the sodium- Received: 21 November 2017 Accepted: 14 June 2018 potassium-chloride-cotransporter Published: xx xx xxxx human variants hNKCC1A and hNKCC1B Philip Hampel 1,2, Kerstin Römermann1, Nanna MacAulay 3 & Wolfgang Löscher1,2 The Na+–K+–2Cl− cotransporter NKCC1 plays a role in neuronal Cl− homeostasis secretion and represents a target for brain pathologies with altered NKCC1 function. Two main variants of NKCC1 have been identifed: a full-length NKCC1 transcript (NKCC1A) and a shorter splice variant (NKCC1B) that is particularly enriched in the brain. The loop diuretic bumetanide is often used to inhibit NKCC1 in brain disorders, but only poorly crosses the blood-brain barrier. We determined the sensitivity of the two human NKCC1 splice variants to bumetanide and various other chemically diverse loop diuretics, using the Xenopus oocyte heterologous expression system. Azosemide was the most potent NKCC1 inhibitor (IC50s 0.246 µM for hNKCC1A and 0.197 µM for NKCC1B), being about 4-times more potent than bumetanide. Structurally, a carboxylic group as in bumetanide was not a prerequisite for potent NKCC1 inhibition, whereas loop diuretics without a sulfonamide group were less potent. None of the drugs tested were selective for hNKCC1B vs. hNKCC1A, indicating that loop diuretics are not a useful starting point to design NKCC1B-specifc compounds. Azosemide was found to exert an unexpectedly potent inhibitory efect and as a non-acidic compound, it is more likely to cross the blood-brain barrier than bumetanide. Te Na+–K+–2Cl− cotransporter NKCC1 (encoded by SLC12A2) plays an important role in Cl- uptake in neu- rons both in developing brain and in adult sensory neurons1,2. -
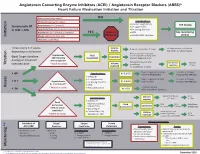
Initiation Titration Assess Monitoring **
Angiotensin Converting Enzyme Inhibitors (ACEI) / Angiotensin Receptor Blockers (ARBS)* Heart Failure Medication Initiation and Titration Bilateral renal artery stenosis NO Moderate/Severe aortic stenosis Considerations: • Baseline cough (ACEi) Hyperkalemia: K+ > 5.2 mmol/L See dosing Symptomatic HF • K+ supplements or LVEF < 40% Renal Dysfunction:Serum creatinine >220 µmol/L • K+ sparing diuretics Hypotension: SBP < 90 mmHg or symptoms YES Refer to • MRA See monitoring Initiation Allergy: angioedema, hives, rash Physician • NSAIDS/COX2 inhibitors section Intolerance: cough (ACEi) Titrate every 1-3 weeks, Volume Reduce/hold diuretic x 2-3 days No improvement, hold/reduce depending on tolerance deplete ACEI/ARB x 1-2 wks & reassess Reassess diuretic dose/other Hypotension Fluid non-essential BP lowering meds Goal: Target dose(see Euvolemic SBP<90mmHg Assessment Consider staggering doses dosing) or maximum with symptoms* Reduce/hold dose of other See Diuretic Reassess Titration * Watch for trends Volume tolerated dose vasodilators algorithm 1-2 wks overload +/- ACEI/ARB x 1-2 weeks Stop K+ supplements, reduce/ Serum K+ in 3-5 days, Considerations: • BP K+ 5.2-5.5 hold MRA (if applicable) Reassess ACEI/ARB dose • Dietary K+ • K+ supplements Stop K+ supplements, MRA. Serum K+ in 2-3 days, • K + Hyperkalemia • K+ sparing diuretics K+ 5.6-6.0 hold ACEI/ARB Reassess ACEI/ARB dose K+ > 5.2 mmol/L* • MRA Assess * Watch for trends • Renal dysfunction Treat hyperkalemia • Scr K+ > 6.0 Refer to MD/NP +/- send to ED Reduce/hold diuretic Scr in Considerations: -

Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax)
Caffeine and Energy Boosting Drugs: Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax) www.ctclearinghouse.org A Library and Resource Center on Alcohol, Tobacco, Other Drugs, Mental Health and Wellness What are energy drinks? you. You wouldn't use Mountain Dew as a sports Energy drinks are beverages like drink. And a drink like Red Bull and vodka is Red Bull, Venom, Adrenaline Rush, more like strong coffee and whisky than 180, ISO Sprint, and Whoopass, anything else. which contain large doses of caffeine and other legal stimulants What happens when energy drinks are like ephedrine, guarana, and ginseng. combined with alcohol? Energy drinks may contain as much as 80 mg. Energy drinks are also used as mixers with of caffeine, the equivalent of a cup of coffee. alcohol. This combination carries a number of Compared to the 37 mg. of caffeine in a dangers: Mountain Dew, or the 23 mg. in a Coca-Cola Classic, that's a big punch. These drinks are • Since energy drinks are stimulants and marketed to people under 30, especially to alcohol is a depressant, the combination of college students, and are widely available both effects may be dangerous. The stimulant on and off campus. effects can mask how intoxicated you are and prevent you from realizing how much Are there short-term dangers to drinking alcohol you have consumed. Fatigue is one energy drinks? of the ways the body normally tells Individual responses to caffeine vary, and someone that they've had enough to drink. these drinks should be treated carefully • The stimulant effect can give the person the because of how powerful they are. -

Drug Classes Other Diuretics
DRUG CLASSES OTHER DIURETICS Loop diuretics and potassium sparing diuretics have limited roles in the management of hypertension although accumulating evidence suggests that potassium-sparing diuretics may have an important place in apparently resistant hypertension. LOOP DIURETICS Examples Bumetamide Furosemide Torasemide Mechanism of action Loop diuretics act on the nephron mainly in the thick ascending links of the Loop of Henle. Although loop diuretics have diuretic efficacy greater than that of thiazide or thiazide-like agents, effects on blood pressure are relatively brief and reflex stimulation of the renin-angiotensin system tends to counter the fall in blood pressure. Pharmacokinetics Loop diuretics are well absorbed after oral administration. Elimination is largely by the renal route with a small contribution from liver metabolism. Like thiazide and thiazide- like diuretics, the renal effects depend on access to the luminal side of the renal tubule; unlike thiazide and thiazide-like diuretics, loop diuretics retain some efficacy in renal impairment. Adverse effects As with thiazide and thiazide-like diuretics, these are mainly metabolic Hypokalaemia is even less common than with low-dose thiazides or thiazide-like diuretics Hyperglycaemia is less marked than with thiazide or thiazide-like diuretics 1 Urate retention risk of acute gout Hypocalcaemia due to increased renal clearance of calcium small risk of ureteric calculi Practical issues Bumetamide and furosemide have a rapid onset and short duration of action. Twice daily dosing is more effective in lowering blood pressure. However, persistent diuresis may limit utility. Torasemide has a longer duration of action and can be given once daily but metabolic complications, particularly hypokalaemia, are more marked than with other loop- diuretics. -

The Necessity and Effectiveness of Mineralocorticoid Receptor Antagonist in the Treatment of Diabetic Nephropathy
Hypertension Research (2015) 38, 367–374 & 2015 The Japanese Society of Hypertension All rights reserved 0916-9636/15 www.nature.com/hr REVIEW The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy Atsuhisa Sato Diabetes mellitus is a major cause of chronic kidney disease (CKD), and diabetic nephropathy is the most common primary disease necessitating dialysis treatment in the world including Japan. Major guidelines for treatment of hypertension in Japan, the United States and Europe recommend the use of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, which suppress the renin-angiotensin system (RAS), as the antihypertensive drugs of first choice in patients with coexisting diabetes. However, even with the administration of RAS inhibitors, failure to achieve adequate anti-albuminuric, renoprotective effects and a reduction in cardiovascular events has also been reported. Inadequate blockade of aldosterone may be one of the reasons why long-term administration of RAS inhibitors may not be sufficiently effective in patients with diabetic nephropathy. This review focuses on treatment in diabetic nephropathy and discusses the significance of aldosterone blockade. In pre-nephropathy without overt nephropathy, a mineralocorticoid receptor antagonist can be used to enhance the blood pressure-lowering effects of RAS inhibitors, improve insulin resistance and prevent clinical progression of nephropathy. In CKD categories A2 and A3, the addition of a mineralocorticoid receptor antagonist to an RAS inhibitor can help to maintain ‘long-term’ antiproteinuric and anti-albuminuric effects. However, in category G3a and higher, sufficient attention must be paid to hyperkalemia. Mineralocorticoid receptor antagonists are not currently recommended as standard treatment in diabetic nephropathy.