What Are the Differences Between Fish, Sharks and Whales?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

STORRE Veral Et Al. Tilapia Locomotor Activity .Pdf
CIRCADIAN RHYTHMS OF LOCOMOTOR ACTIVITY IN THE NILE TILAPIA OREOCHROMIS NILOTICUS Luisa María Veraa, Louise Cairnsa, Francisco Javier Sánchez-Vázquezb, Hervé Migauda* a Reproduction and Genetics Group, Institute of Aquaculture, University of Stirling. Stirling, UK. b Department of Physiology, Faculty of Biology, University of Murcia, 30100 Murcia, Spain. Running title: Circadian rhythms in tilapia *Corresponding author: Dr. Hervé Migaud Institute of Aquaculture, University of Stirling FK9 4LA, Stirling, UK Tel. 0044 1786 467886 Fax. 0044 1786 472133 E-mail: [email protected] 1 ABSTRACT The Nile tilapia behavioural rhythms were investigated to better characterize its circadian system. To do so, the locomotor activity patterns of both male and female tilapia reared under a 12: 12-h light-dark (LD) cycle were studied, as well as the existence of endogenous rhythmicity under free-running conditions (DD and 45-min LD pulses) in males. When exposed to an LD cycle, the daily pattern of activity differed between individuals: some fish were diurnal, some nocturnal and a few displayed an arrhythmic pattern. This variability would be typical of the plastic circadian system of fish and reproductive events clearly affected the behavioural rhythms of female tilapia, a mouthbrooder teleost species. Under DD, 50% (6 out of 12) of male fish showed circadian rhythms with an average period (tau) of 24.1 ± 0.2 h whereas under the 45- min LD pulses 58% (7 out of 12) of fish exhibited free-running activity rhythms and tau was 23.9 ± 0.5 h. However, interestingly in this case activity was always confined to the dark phase. -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Snakeheadsnepal Pakistan − (Pisces,India Channidae) PACIFIC OCEAN a Biologicalmyanmar Synopsis Vietnam
Mongolia North Korea Afghan- China South Japan istan Korea Iran SnakeheadsNepal Pakistan − (Pisces,India Channidae) PACIFIC OCEAN A BiologicalMyanmar Synopsis Vietnam and Risk Assessment Philippines Thailand Malaysia INDIAN OCEAN Indonesia Indonesia U.S. Department of the Interior U.S. Geological Survey Circular 1251 SNAKEHEADS (Pisces, Channidae)— A Biological Synopsis and Risk Assessment By Walter R. Courtenay, Jr., and James D. Williams U.S. Geological Survey Circular 1251 U.S. DEPARTMENT OF THE INTERIOR GALE A. NORTON, Secretary U.S. GEOLOGICAL SURVEY CHARLES G. GROAT, Director Use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Geological Survey. Copyrighted material reprinted with permission. 2004 For additional information write to: Walter R. Courtenay, Jr. Florida Integrated Science Center U.S. Geological Survey 7920 N.W. 71st Street Gainesville, Florida 32653 For additional copies please contact: U.S. Geological Survey Branch of Information Services Box 25286 Denver, Colorado 80225-0286 Telephone: 1-888-ASK-USGS World Wide Web: http://www.usgs.gov Library of Congress Cataloging-in-Publication Data Walter R. Courtenay, Jr., and James D. Williams Snakeheads (Pisces, Channidae)—A Biological Synopsis and Risk Assessment / by Walter R. Courtenay, Jr., and James D. Williams p. cm. — (U.S. Geological Survey circular ; 1251) Includes bibliographical references. ISBN.0-607-93720 (alk. paper) 1. Snakeheads — Pisces, Channidae— Invasive Species 2. Biological Synopsis and Risk Assessment. Title. II. Series. QL653.N8D64 2004 597.8’09768’89—dc22 CONTENTS Abstract . 1 Introduction . 2 Literature Review and Background Information . 4 Taxonomy and Synonymy . -

O2 Secretion in the Eye and Swimbladder of Fishes
1641 The Journal of Experimental Biology 209, 1641-1652 Published by The Company of Biologists 2007 doi:10.1242/jeb.003319 Historical reconstructions of evolving physiological complexity: O2 secretion in the eye and swimbladder of fishes Michael Berenbrink School of Biological Sciences, The University of Liverpool, Biosciences Building, Crown Street, Liverpool, L69 7ZB, UK e-mail: [email protected] Accepted 12 March 2007 Summary The ability of some fishes to inflate their compressible value of haemoglobin. These changes predisposed teleost swimbladder with almost pure oxygen to maintain neutral fishes for the later evolution of swimbladder oxygen buoyancy, even against the high hydrostatic pressure secretion, which occurred at least four times independently several thousand metres below the water surface, has and can be associated with increased auditory sensitivity fascinated physiologists for more than 200·years. This and invasion of the deep sea in some groups. It is proposed review shows how evolutionary reconstruction of the that the increasing availability of molecular phylogenetic components of such a complex physiological system on a trees for evolutionary reconstructions may be as important phylogenetic tree can generate new and important insights for understanding physiological diversity in the post- into the origin of complex phenotypes that are difficult to genomic era as the increase of genomic sequence obtain with a purely mechanistic approach alone. Thus, it information in single model species. is shown that oxygen secretion first evolved in the eyes of fishes, presumably for improved oxygen supply to an Glossary available online at avascular, metabolically active retina. Evolution of this http://jeb.biologists.org/cgi/content/full/210/9/1641/DC1 system was facilitated by prior changes in the pH dependence of oxygen-binding characteristics of Key words: oxygen secretion, Root effect, rete mirabile, choroid, haemoglobin (the Root effect) and in the specific buffer swimbladder, phylogenetic reconstruction. -

Bony Fish Guide
This guide will help you to complete the Bony Fish Observation Worksheet. Bony Fish Guide Fish (n.) An ectothermic (cold-blooded) vertebrate (with a backbone) aquatic (lives in water) animal that moves with the help of fins (limbs with no fingers or toes) and breathes with gills. This definition might seem very broad, and that is because fish are one of the most diverse groups of animals on the planet—there are a lot of fish in the sea (not to mention rivers, lakes and ponds). In fact, scientists count at least 32,000 species of fish—more than any other type of vertebrate. Fish are split into three broad classes: Jawless Fish Cartilaginous Fish Bony Fish (hagfish, lampreys, etc.) (sharks, rays, skates, etc.) (all other fish) This guide will focus on the Bony Fish. There are at least 28,000 species of bony fish, and they are found in almost every naturally occurring body of water on the planet. Bony fish range in size: • Largest: ocean sunfish (Mola mola), 11 feet, over 5,000 pounds • Smallest: dwarf pygmy goby (Pandaka pygmaea), ½ inch, a fraction of an ounce (This image is life size.) The following guide will help you learn more about the bony fish you can find throughout the New England Aquarium. Much of the guide is keyed to the Giant Ocean Tank, but can be applied to many kinds of fish. Even if you know nothing about fish, you can quickly learn a few things: The shape of a fish’s body, the position of its mouth and the shape of its tail can give you many clues as to its behavior and adaptations. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -

Doublespot Acara (Aequidens Pallidus) Ecological Risk Screening Summary
Doublespot Acara (Aequidens pallidus) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, web version – 03/29/2018 Photo: Frank M Greco. Licensed under Creative Commons BY 3.0 Unported. Available: https://commons.wikimedia.org/wiki/File:Aequidens_pallidus.jpg. (August 2017). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2015): “South America: Amazon River basin, in the middle and lower Negro River, Uatumã, Preto da Eva, and Puraquequara rivers.” Status in the United States No records of Aequidens pallidus in the United States found. 1 Means of Introductions in the United States No records of Aequidens pallidus in the United States found. Remarks No additional remarks. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2015): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Osteichthyes Class Actinopterygii Subclass Neopterygii Infraclass Teleostei Superorder Acanthopterygii Order Perciformes Suborder Labroidei Family Cichlidae Genus Aequidens Species Aequidens pallidus (Heckel, 1840)” From Eschmeyer et al. (2017): “pallidus, Acara Heckel [J. J.] 1840:347 […] [Annalen des Wiener Museums der Naturgeschichte v. 2] Rio Negro of Rio Amazonas, South America. Holotype (unique): NMW 33678. •Valid as Aequidens pallidus (Heckel 1840) -- (Kullander in Reis et al. 2003:608 […]). Current status: Valid as Aequidens pallidus (Heckel 1840). Cichlidae: Cichlinae.” Size, Weight, and Age Range From Froese and Pauly (2015): “Max length: 14.3 cm SL male/unsexed; [Kullander 2003]” “Maximum length 20.0 cm TL [Stawikowski and Werner 1998].” 2 Environment From Froese and Pauly (2015): “Freshwater; benthopelagic; pH range: 6.5 - 7.5; dH range: ? - 10. -

Notes on the Swim-Bladder Physiology of Cod (Gadus Morhua) Investigated from the Underwater Laboratory "Helgoland"
Helgol~inder wiss. Meeresunters. 29, 460-463 (1977) Notes on the swim-bladder physiology of cod (Gadus morhua) investigated from the underwater laboratory "Helgoland" G. SUNDNES, B. GULLIKSEN ~X~ J. MORK Biological stasjon; Trondheim, Norway ABSTRACT: In situ sampling of gas from cod swim-bladders took place during a fortnight's saturation mission with the underwater laboratory "Helgoland" in May-June I975. These samples were compared to those done by the conventional method of transporting the fish to the surface for sampling. Based upon these in-situ measurements, the mean O2-concentration was 55.7 °/0 in buoyant cod at 15 m depth. Repeated sampling of the same fish showed a change in gas composition. Compared to the conventional method of transporting fish for sampling to the surface, in-situ sampling gave results with less variation, and indicated that surface-sampling does not give the correct gas composition of buoyant fish at depth of catch. INTRODUCTION Physiological investigations of fish swim-bladder have usually been performed near the surface, i.e. at about one atmosphere pressure. It means that fish were usually brought to the surface from their natural habitat of high water pressure to reduced pressure before experiments were performed and samples taken. Even in experiments with fish in pressure chambers, the gas samples of the swim-bladder were taken at one atmosphere pressure. Gas sampIes from fish at the depth of buoyancy have rarely been reported. The development of the underwater laboratory has given marine biologists a better opportunity to work under hydrostatic pressure whereby fish are kept under "natural" experimental conditions. -

Alcolapia Grahami ERSS
Lake Magadi Tilapia (Alcolapia grahami) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, March 2015 Revised, August 2017, October 2017 Web Version, 8/21/2018 1 Native Range and Status in the United States Native Range From Bayona and Akinyi (2006): “The natural range of this species is restricted to a single location: Lake Magadi [Kenya].” Status in the United States No records of Alcolapia grahami in the wild or in trade in the United States were found. The Florida Fish and Wildlife Conservation Commission has listed the tilapia Alcolapia grahami as a prohibited species. Prohibited nonnative species (FFWCC 2018), “are considered to be dangerous to the ecology and/or the health and welfare of the people of Florida. These species are not allowed to be personally possessed or used for commercial activities.” Means of Introductions in the United States No records of Alcolapia grahami in the United States were found. 1 Remarks From Bayona and Akinyi (2006): “Vulnerable D2 ver 3.1” Various sources use Alcolapia grahami (Eschmeyer et al. 2017) or Oreochromis grahami (ITIS 2017) as the accepted name for this species. Information searches were conducted under both names to ensure completeness of the data gathered. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing According to Eschmeyer et al. (2017), Alcolapia grahami (Boulenger 1912) is the current valid name for this species. It was originally described as Tilapia grahami; it has also been known as Oreoghromis grahami, and as a synonym, but valid subspecies, of -
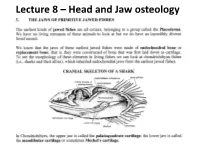
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Invasive Species of the Pacific Northwest
Invasive Species of the Pacific Northwest: Green Sunfish Lepomis cyanellus Derek Arterburn FISH 423: Olden 12.5.14 Figure 1: Adult Green sunfish Lepomis cyanellus . Photo from http://www.freshwater-fishing- news.com/fish-species-north -america/green-sunfish/ Classification Lepomis cyanellus may have a few teeth, Order: Perciformes which can be found on the tongue. Family: Centrarchidae Additional distinguishing marks are the 7-12 Genus: Lepomis parallel diffused dark bars running ventral to Species: cyanellus dorsal along the side of L. cyanellus, and the bluish-green pattern. The bluish-green Identification coloration takes place on the mainly black/dark brown/olive body, composed of Adult Green Sunfish, Lepomis ctenoid scales, which fades to a lighter cyanellus, commonly reach a total length of ventral color. The dark sides of L. cyanellus 31cm, with juveniles ranging from 12-15cm. are contrast with a yellow/cream ventral Adult Green Sunfish have been known to coloration (Cockerell 1913). The thick reach a maximum weight of one kilogram caudal peduncle is without an adipose fin, (2.2lbs). L. cyanellus is a deep bodied, and the peduncle runs to a rounded, slightly laterally compressed species, with a lateral forked, homocercal caudal fin. The paired line running from the operculum to the fins on Lepomis cyanellus are derived in caudal peduncle. The posterior of the orientation. The Green Sunfish has lateral operculum has a characteristic dark spot placement of the pectoral fins with vertical relatively the same size as the eye, and the insertion, anterior pelvic fins, and spines same size spot may also be found at the base found on the anal and dorsal fins. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes