Superconducting and Structural Properties Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COI QUERY Disclaimer
COI QUERY Country of Origin Pakistan Main subject Situation in Pakistan-administered Kashmir Question(s) 1. General information Historical background Population and ethnic groups Returnees in Kashmir and Punjab Religious demography 2. Political situation 3. Human rights situation General overview Ethnic conflict Sectarian conflict 4. Security situation Conflict-related violence Line of control violations Cross-border attacks Examples of cross-border violence in 2020 Armed groups Hizb-ul-Mujahideen (HM) Jaish-e Muhammad (JeM) Lashkar-e Taiba (LeT) Harakat ul-Mujahidin (HuM) Date of completion 6 October 2020 Query Code Q27-2020 Contributing EU+ COI -- units (if applicable) Disclaimer This response to a COI query has been elaborated according to the EASO COI Report Methodology and EASO Writing and Referencing Guide. The information provided in this response has been researched, evaluated and processed with utmost care within a limited time frame. All sources used are referenced. A quality review has been performed in line with the above mentioned methodology. This document does not claim to be exhaustive neither conclusive as to the merit of any particular claim to international protection. If a certain event, person or organisation is not mentioned in the report, this does not mean that the event has not taken place or that the person or organisation does not exist. Terminology used should not be regarded as indicative of a particular legal position. 1 The information in the response does not necessarily reflect the opinion of EASO and makes no political statement whatsoever. The target audience is caseworkers, COI researchers, policy makers, and decision making authorities. The answer was finalised on 6 October 2020. -

According to the Syllabus of University of Azad Jammu & Kashmir
LLB FIVE YEARS DEGREE PROGRAMME NOTES According to the Syllabus of University of Azad Jammu & Kashmir Muzaffarabad and Other Public, Private Sector Universities of Pakistan Prepared By Advocate Muhammad Adnan Masood Joja Sardar Javed Zahoor Khan (Advocate) CITI Law College Rawalakot 05824-442207, 444222, 0332-4573251, 051-4852737 Near CMH Rawalakot AJK Web:-www.clc.edu.pk Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 1 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 2 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 3 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 4 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 5 FUNCTIONAL ENGLISH Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 6 Parts of Speech NOUNS A noun is the word that refers to a person, thing or abstract idea. A noun can tell you who or what. There are several different types of noun: - There are common nouns such as dog, car, chair etc. Nouns that refer to things which can be counted (can be singular or plural) are countable nouns. Nouns that refer to some groups of countable nouns, substances, feelings and types of activity (can only be singular) are uncountable nouns. Nouns that refer to a group of people or things are collective nouns. Nouns that refer to people, organizations or places are proper nouns, only proper nouns are capitalized. -

Evaluation of Covid-19 Pandemic in Azad Jammu And
Open Access Original Article Evaluation of COVID-19 Pandemic Pak Armed Forces Med J 2020; 70 (6): 1913-18 EVALUATION OF COVID-19 PANDEMIC IN AZAD JAMMU AND KASHMIR PAKISTAN Qaesar Nazeer, Tahir Sardar*, Syed Fawad Mashhadi* WHO AJK Muzaffarabad Pakistan, *Army Medical College/National University of Medical Sciences (NUMS) Rawalpindi Pakistan ABSTRACT Objective: To examine mitigation measures in Azad, Jammu and Kashmir and compare their effectiveness to rest of Pakistan. Study Design: Prospective observational study. Place and Duration of Study: Province of Azad, Jammu and Kashmir, from Mar to Aug 2020. Methodology: This study was conducted by Provincial Disease Surveillance Department of World Health Organization at Azad, Jammu and Kashmir Muzaffarabad after getting approval from Health Department. Data were collected from different hospitals of Azad Jammu and Kashmir as per a specially designed proforma. Results: No significant variation from national averages was observed, adapting mitigation measures and lockdown were success in decreasing epidemic curve. Conclusion: The easing of the lockdown policy resulted in widespread outbreak in the province and non- compliance with infection control best practices by members of the community, similar to rest of Pakistan. Keywords: Mitigation measures, Public health, Risk communication. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. INTRODUCTION 1958 declaring COVID-19 as disease of Public COVID-19 is a novel respiratory virus which Emergency in the wake of an emergent situation critically affected multi-organ systems in infected resulting from the outbreak of the Corona virus individuals1,2. -

A First Ever Detailed Ecological Exploration of the Western Himalayan Forests of Sudhan Gali and Ganga Summit, Azad Jammu and Kashmir, Pakistan - 15477
Khan et al.: A first ever detailed ecological exploration of the Western Himalayan forests of Sudhan Gali and Ganga Summit, Azad Jammu and Kashmir, Pakistan - 15477 - A FIRST EVER DETAILED ECOLOGICAL EXPLORATION OF THE WESTERN HIMALAYAN FORESTS OF SUDHAN GALI AND GANGA SUMMIT, AZAD JAMMU AND KASHMIR, PAKISTAN KHAN, A. M.1,2* – QURESHI, R.2 – SAQIB, Z.3 – MUNIR, M.4 – SHAHEEN, H.5 – HABIB, T.5 – DAR, M. E. U. I.5 – FATIMAH, H.6 – AFZA, R.7 – HUSSAIN, M. A.8 1Department of Botany, Govt. Hashmat Ali Islamia Degree College, Rawalpindi, Pakistan 2Department of Botany, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan 3Department of Environmental Science, International Islamic University, Islamabad, Pakistan 4Department of Botany, University of Animal & Plant Sciences, Ravi Campus, Pattoki, Punjab, Pakistan 5Department of Botany, University of Azad Jammu & Kashmir, Muzaffarabad, Pakistan 6Department of Biology & Environmental Science, Allama Iqbal Open University, H-8 Islamabad, Pakistan 7Department of Botany, Hazara University Mansehra, Khyber Pakhtunkhwa, Pakistan 8Department of Biotechnology, Mirpur University of Science & Technology, 10250 Mirpur, AJK, Pakistan *Corresponding author e-mail: [email protected]; phone: +92-33-3521-7235 (Received 22nd Jul 2019; accepted 16th Oct 2019) Abstract. The baseline ecological exploratory studies of floristically rich areas like the western Himalaya are very important to determine the diversity variations. Based on research gaps detected in ecological literature of the western Himalayan forests of Sudhan Gali and Ganga summit, the study area is revisited. Plots data was collected during 2016, and advanced multivariate statistical tools were used to analyze it. Some of multivariate tools including permutation test, indicator species analysis and variation partitioning (partial CCA) were used for the first time in the area. -

Report Pakistan
OPTIFOOD ANALYSIS REPORT PAKISTAN August 2018 Copyright © UNICEF Pakistan & Ministry of National Health Services, Regulations and Coordination Prepared by: Julie Peletier Design: Human Design Studios OPTIFOOD OPTIFOOD ANALYSIS REPORT PAKISTAN REPORT 2018 Acknowledgments I would like to thank the Ministry of National Health Services, Regulation and Coordination and the UNICEF- Pakistan team for their support to the Optifood study and especially to Dr Saba Shuja for her day-to-day communication, coordination and guidance. Furthermore, I would like to thank Pablo Rodriguez for his guidance. Great appreciation goes to the Research and Development Solutions (RADS) team, and especially to Dr Adnan A. Khan and Safoora Malik for mobilizing the team of data collectors and supervisors. This analysis would not have been possible without the willing help of the women who participated in the interviews which is greatly appreciated. Lastly, I would like to thank all persons not mentioned by name who contributed to the success of this project. Julie Peletier Disclaimer: The study has been made possible with the financial assistance of the Department for International Development (DFID), United Kingdom. The views expressed herein should not be taken, in any way, to reflect the official opinion of the DFID. 3 OPTIFOOD REPORT OPTIFOOD ANALYSIS REPORT PAKISTAN 2018 Foreword Appropriate feeding practices are essential for the nutritional status, growth, development and survival of infants and young children. Infants should be exclusively breastfed for the first six months of life, and thereafter receive nutritionally-adequate and safe complementary foods, while breastfeeding should continue up to at least two years of age. Poor or suboptimal breastfeeding practices are an important determinant of all forms of undernutrition, especially during the first 1,000 days of a child’s life. -

In Azad Jammu and Kashmir, Pakistan
Journal of Wildlife and Ecology. (2019). 3(4):1-5 W E Research Paper Open Access Distribution of Mackinnon’s Wolf Snake (Lycodon mackinnoni Wall, 1906) in Azad Jammu and Kashmir, Pakistan Rabia Lal1*, Muhammad Samar Hussain Khan2, Sadia Nazer1, Mehwish Altaf1, Samra Iram Farooq1, Laraib Safdar1, Maryam Faiz1 and Ali Muhammad3 1. Department of Zoology, Women University of AJK, Bagh, Pakistan 2. Ministry of Climate Change, Islamabad, Pakistan 3. Department of Zoology, University of Poonch, Rawalakot, Pakistan *Corresponding author: [email protected] Peer Reviewed ABSTRACT Total 10,450 species of reptiles are reported in the world; while 195 species are present in Pakistan; Lycodon mackinnoni known as Himalayan wolf snake; belong to Citation: Lal, R., M.S.H. Colubridae family. The main objective of the study was to Khan, S. Nazer, M. Altaf, S.I. explore the distribution of the species. The sample collected Farooq, L. Safdar, M. Faiz, A. from the Dhirkot (district Bagh), Hattian Bala, Sudhnoti, Muhammad. 2019. Distribution of Mackinnon’s Dhirkot (district Bagh), Poonch, Haveli and Kotli in AJK. Wolf Snake (Lycodon 9 AJ&K is present in humid region; a lot of variation in mackinnoni Wall, 1906) in humidity in various parts with differences in altitudes. The Azad Jammu and Kashmir, Pakistan Journal of Wildlife winters are severely cold (04ºC) while the summer is and Ecology. 3(4): 1-5. moderate (37ºC); snowfall has been recorded at higher elevations. The average rainfall was recorded as 150mm. Received: 11, 10, 2019 Accepted: 07, 11, 2019 The data were collected from November 2016 to April 2019 Published: 01, 12, 2019 from the selected study areas. -

Trail of Tragedy 5.Pdf
Trail of Tragedy-5 Chronology of Disaster in Pakistan 2014 Trail of Tragedy - 5 Chronology of Disaster in Pakistan 2014 Contents Acronyms iv Acknowledgment v Background vi News Items 2014 1 Editorials - Articles 99 Strengthening Participatory Organization (SPO) iii Trail of Tragedy - 5 Chronology of Disaster in Pakistan 2014 Acronyms FFC Federal Flood Commission IUCN International Union for Conservation of Nature LBOD Left Bank Outfall Drain MNV Main Nara Valley NADRA National Database and Registration Authority NDMA National Disaster Management Authority NNS National Nutrition Survey PDMA Provincial Disaster Management Authority PMD Pakistan Meteorological Department RBOD-I Right Bank Outfall Drain SIDA Sindh Irrigation and Drainage Authority SUPARCO Pakistan Space and Upper Atmosphere Research Commission UN United Nations UNESCO United Nations Educational, Scientific and Cultural Organization WFP World Food Programme Strengthening Participatory Organization (SPO) iv Trail of Tragedy - 5 Chronology of Disaster in Pakistan 2014 Acknowledgment It is acknowledged that this chronology of disaster related to the floods in Sindh in 2014 has been compiled using the online archives of major national newspapers: Dawn at www.dawn. com, The News at www. thenews.com.pk and Express Tribune. SPO presents this chronology through the courtesy of these two media outlets. However, this data does not represent a hundred percent coverage of the disaster and response that have been reported. Editorials, news items and letters to Editors, included in this chronology are related to heavy Rains, Floods, Human security, Vulnerable Groups, Food Security, Livelihood and Aid & Aid effectiveness. SPO does not take the responsibility either for the authenticity of contents of news stories included in this report, or for the treatment of the flood disaster by the media. -

Perspective from Poonch and Kotli Region of AJK
Journal of Political Studies, Vol. 24, Issue - 2, 2017, 535:562 Living on the frontlines: Perspective from Poonch and Kotli region of AJK Shaheen Akhtar* Abstract Kashmir conflict deeply impacts the lives of the people living along the 740km Line of Control (LoC), dividing former state of Jammu and Kashmir. The people living in the in Kotli and Poonch districts are extremely vulnerable to volatile conditions of the LoC which adversely affect their physical security, livelihood, socio-cultural life, educational development, psychological health and wellbeing. This study evaluates the impact of Kashmir conflict and escalation of tension on the LoC in the 1990s, the impact of 2003 ceasefire and recent LoC tension on the lives of the people living in villages on the zero line in Kotli and Poonch districts. It argues that human dimension of the Kashmir conflict should be recognized, ceasefire on the LoC institutionalized and stalemated political dialogue resumed to revive political process on resolution of Kashmir dispute. Key words: Kashmir conflict, Line of Control, Poonch, Kotli, impact of firing, shelling, 2003 ceasefire Introduction The people living along the LoC are extremely vulnerable to militarization and volatility of the LoC which adversely affect their physical security, livelihood, socio- cultural life, educational development, psychological health and wellbeing. The LoC became a battle zone during India-Pakistan wars of 1948, 1965 and 1971 and remained explosive in the warlike situations such as in 1990, in 1999 during Kargil crisis and in 2001-2002 following military standoff. With uprising in Indian held Jammu & Kashmir (IHK) in the 1990s, the incidence of firing and shelling on the LoC went up resulting in killing, injuring and displacement of a large number of civilian populations inhabiting the bordering areas of Azad Jammu and Kashmir (AJK). -

Ideology of Pakistan and the Old Civilizations Including the Indus Valley and Gandhara
PAKISTAN STUDIES (Compulsory) For BS Course Code: (9374) Units: 1–9 Department of Pakistan Studies Faculty of Social Sciences and Humanities ALLAMA IQBAL OPEN UNIVERSITY, ISLAMABAD (ALL RIGHTS RESERVED WITH THE PUBLISHER) 1st Edition ........................................... 2019 Quantity ............................................. Price ................................................... Publishing Supervisor ........................ Printer ................................................ Allama Iqbal Open University, Islamabad. Publisher ............................................ Allama Iqbal Open University, Islamabad. Course Development Coordinator: ..... Dr. Khalid Mahmood Editor: ................................................ Ms. Humera Ejaz ii COURSE TEAM Chairman: Prof. Dr. Samina Awan Course Coordinator: Dr. Khalid Mahmood Writers: 1. Prof. Dr. Muhammad Reza Kazimi 2. Dr. Khalid Mahmood 3. Mudassir Jattala 4. Sayed Akmal Shah \ Reviewers: 1. Prof. Dr. Samina Awan 2. Prof. Dr. Sayed Jaffar Ahmed 3. Prof. Dr. Fazal-i-Rahim Marwat 4. Prof. Dr. Anwar Shaheen 5. Dr. Sajid Mahmood Awan 6. Dr. Umar Hayat 7. Dr. Kishwar Sultana 8. Dr. Kausar Parveen Editor: Ms. Humera Ejaz Layout & Design: Malik Mateen Ishfaq iii FOREWORD Allama Iqbal Open University has the honour to present BS Programs in various faculties for those who are deprived from regular education due to their compulsions. It is obviously your own institution that provides you the education facility at your door step. Pakistan, our beloved country had been facing numerous issues since its creation. The initial days were very tough for the newly state but with the blessings of Allah Almighty, it made progress day by day. However, due to conspiracy of external powers and some weaknesses of our leaders, the internal situation of East Pakistan rapidly changed and the end was painful as we lost not only the land but also our Bengali brothers. After the war of 1971, the people and leaders of Pakistan were forced to rethink the future of the remaining country. -
Pakistan Security Report 2020
Conflict and Peace Studies VOLUME 13 Jan - June 2021 NUMBER 1 PAKISTAN SECURITY REPORT 2020 PAK INSTITUTE FOR PEACE STUDIES (PIPS) A PIPS Research Journal Conflict and Peace Studies Copyright © PIPS 2021 All Rights Reserved No part of this journal may be reproduced in any form by photocopying or by any electronic or mechanical means, including information storage or retrieval systems, without prior permission in writing from the publisher of this journal. Editorial Advisory Board Khaled Ahmed Dr. Muhammad Khalid Masud Consulting Editor, Former Chairman of the Council of Islamic The Friday Times, Lahore, Pakistan. Ideology in Pakistan. Prof. Dr. Saeed Shafqat Dr. Catarina Kinnvall Director, Centre for Public Policy and Governance, Department of Political Science, Forman Christian College, Lahore, Pakistan. Lund University, Sweden. Marco Mezzera Dr. Adam Dolnik Senior Adviser, Norwegian Peacebuilding Resource Professor of Counterterrorism, George C. Centre / Norsk Ressurssenter for Fredsbygging, Marshall European Center for Security Studies, Norway. Germany. Prof. Dr. Syed Farooq Hasnat Tahir Abbas Pakistan Study Centre, University of the Punjab, Professor of Sociology, Fatih University, Lahore, Pakistan. Istanbul, Turkey. Anatol Lieven Rasul Bakhsh Rais Professor, Department of War Studies, Professor, Political Science, King's College, London, United Kingdom. Lahore University of Management Sciences Lahore, Pakistan. Peter Bergen Dr. Tariq Rahman Senior Fellow, New American Foundation, Dean, School of Education, Beaconhouse Washington D.C., USA. National University, Lahore, Pakistan. Pak Institute for Peace ISSN 2072-0408 ISBN 978-969-9370-37-3 (PIPS) Studies Price: Rs 600.00 Post Box No. 2110, US$ 30.00 Islamabad, Pakistan The views expressed are the authors' +92-51-8359475-6 own and do not necessarily reflect any www.pakpips.com, positions held by the institute. -

Contingency Programme Document for WASH Response for 100,000 Disaster Affected People of Azad Jammu Kashmir (AJK)
Sep 30, 2021, 5:24:35 PM Call for Expression of Interest Contingency Programme Document for WASH Response for 100,000 disaster affected people of Azad Jammu Kashmir (AJK) CEF/PAK/2021/015 1 Timeline Posted Jun 2, 2021 Clarification Request Deadline Jun 14, 2021 Application Deadline Jun 21, 2021 Notification of Results Jul 12, 2021 Start Date Aug 1, 2021 End Date Dec 31, 2022 2 Locations A Pakistan a Muzaffarabad District b Neelum District c Jhelum Valley District d Bagh District e Haveli District f Sudhanoti District g Poonch District 3 Sector(s) and area(s) of specialization A WASH and Environment a WASH in emergencies 4 Issuing Agency UNICEF 5 Project Background UNICEF works with the government and other partners for ensuring that most vulnerable and marginalized children and women have access to safe drinking water, improved sanitation facilities and are practicing safe hygiene, by creating enabling environment where civil society duty bearers with strengthened capacity are reducing disaster risks, as well as planning, preparing, and effectively responding to disasters, in accordance with UNICEF Core Commitments to Children in Humanitarian Action. Within the framework set out in UNICEF’s country programme 2018-22, UNICEF will be extending its interventions in case of any unforeseen emergency in AJK, to provide WASH services to the affected communities. UNICEF seeks for partnership with CSO for ensuring emergency WASH interventions for potential risks particularly floods, earthquakes, pandemics (COVID-19), conflicts and landslides for 100,000 people for all the district of AJK. 6 Expected Results Provision of safe drinking water to 70,000 people through Water trucking including installation of water storage tanks, identification and rehabilitation of water supply schemes, identification and rehabilitation of hand pumps, identification of needs for use of household water treatment options and distribution of Household Water Treatment (HHWT) options and water quality testing. -
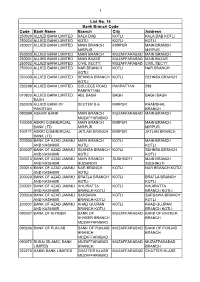
List No. 14 Bank Branch Code
1 List No. 14 Bank Branch Code Code Bank Name Branch City Address 250020 ALLIED BANK LIMITED KALA DAB KOTLI KALA DAB KOTLI 250024 ALLIED BANK LIMITED KOTLI KOTLI KOTLI 250027 ALLIED BANK LIMITED MAIN BRANCH MIRPUR MAIN BRANCH MIRPUR MIRPUR 250030 ALLIED BANK LIMITED MAIN BRANCH MUZAFFARABAD MAIN BRANCH 250031 ALLIED BANK LIMITED MAIN BAZAR MUZAFFARABAD MAIN BAZAR 250032 ALLIED BANK LIMITED CIVIL SECTT: MUZAFFARABAD CIVIL SECTT: 250033 ALLIED BANK LIMITED NAR BRANCH KOTLI NAR BRANCH KOTLI 250038 ALLIED BANK LIMITED SEHNSA BRANCH KOTLI SEHNSA BRANCH KOTLI 250298 ALLIED BANK LIMITED COLLEGE ROAD PAKPATTAN 298 PAKPATTAN 010833 ALLIED BANK LIMITED ABL BAGH BAGH BAGH BAGH BAGH 250029 ALLIED BANK OF SECTER B-5 MIRPUR KHAMBHAL PAKISTAN BRANCH 090099 ASKARI BANK MAIN BRANCH MUZAFFARABAD MAIN BRANCH MUZAFFARABAD 100005 ASKRI COMMERCIAL MAIN BRANCH MIRPUR MAIN BRANCH BANK LTD MIRPUR MIRPUR 100171 ASKRI COMMERCIAL JATLAN BRANCH MIRPUR JATLAN BRANCH BANK LTD 200005 BANK OF AZAD JAMMU MAIN BRANCH KOTLI MAIN BRANCH AND KASHMIR KOTLI KOTLI 200007 BANK OF AZAD JAMMU SEHNSA BRANCH KOTLI SEHNSA BRANCH AND KASHMIR KOTLI KOTLI 200013 BANK OF AZAD JAMMU MAIN BRANCH SUDHNOTI MAIN BRANCH AND KASHMIR SUDHNOTI SUDHNOTI 200014 BANK OF AZAD JAMMU NAR BRANCH KOTLI NAR BRANCH KOTLI AND KASHMIR KOTLI 200020 BANK OF AZAD JAMMU BRATLA BRANCH KOTLI BRATLA BRANCH AND KASHMIR KOTLI KOTLI 200021 BANK OF AZAD JAMMU KHUIRATTA KOTLI KHUIRATTA AND KASHMIR BRANCH KOTLI BRANCH KOTLI 200026 BANK OF AZAD JAMMU SARSAWA KOTLI SARSAWA BRANCH AND KASHMIR BRANCH KOTLI KOTLI 200031 BANK OF AZAD JAMMU KHAD GUJRAN KOTLI KHAD GUJRAN AND KASHMIR BRANCH KOTLI BRANCH KOTLI 090027 BANK OF KHYBER BANK OF MUZAFFARABAD BANK OF KHYBER KHYBER BRANCH BRANCH MUZAFFARABAD 090246 BANK OF PUNJAB BANK OF PUNJAB MUZAFFARABAD BANK OF PUNJAB BRANCH BRANCH MUZAFFARABAD 090072 DUBAI ISLAMIC BANK MUZAFFARABAD MUZAFFARABAD MUZAFFARABAD LIMITED BRANCH BRANCH 222275 HABIB BANK LIMIITED CHATTER KLASS MUZAFFARABAD CHATTER KLASS MUZAFFARABAD 2 List No.