Patterns and Drivers of Long-Term Changes in Breeding Bird Communities in a Global Biodiversity Hotspot in Mexico
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bill Shape As a Generic Character in the Cardinals
BILL SHAPE AS A GENERIC CHARACTER IN THE CARDINALS WALTER J. BOCK ANY genera in birds and other animal groups have been based essentially M upon a single character. This character may be a single morphological feature, such as the presence or absence of the hallux, or it may be a complex of characters which are all closely correlated functionally, such as the bones, muscles, and ligaments of the jaw apparatus. The validity of many of these genera has been questioned in recent years with the general acceptance of the polytypic species concept and the increasing acknowledgment of the grouping service at low taxonomic levels provided by the genus. An example is the North American passerine genus Pyrrhuloxia, which is distinguished from Richmondena essentially on the basis of bill shape. The overall similarity of Pyrrhuloxia sinuata to the two species of Richmondena in morphology and in general life history (Gould, 1961) has led several recent authors to synony- mize Richmondena with Pyrrhuloxia. Other workers have maintained the validity of the generic separation, basing their decision largely on the dif- ference in bill shape. The object of this paper is to ascertain the importance of this difference as a taxonomic character and whether the difference if con- firmed is of generic significance. THE JAW APPARATUS Ridgway (19013624-625) described the bill of P. sinuata (see Figs. 1 and 2) as follows: “Bill very short, thick and deep, with culmen strongly convex and maxillary tomium deeply and angularly incised a little posterior to the middle -

Rivoli's Hummingbird: Eugenes Fulgens Donald R
Digital Commons @ George Fox University Faculty Publications - Department of Biology and Department of Biology and Chemistry Chemistry 6-27-2018 Rivoli's Hummingbird: Eugenes fulgens Donald R. Powers George Fox University, [email protected] Follow this and additional works at: https://digitalcommons.georgefox.edu/bio_fac Part of the Biodiversity Commons, Biology Commons, and the Poultry or Avian Science Commons Recommended Citation Powers, Donald R., "Rivoli's Hummingbird: Eugenes fulgens" (2018). Faculty Publications - Department of Biology and Chemistry. 123. https://digitalcommons.georgefox.edu/bio_fac/123 This Article is brought to you for free and open access by the Department of Biology and Chemistry at Digital Commons @ George Fox University. It has been accepted for inclusion in Faculty Publications - Department of Biology and Chemistry by an authorized administrator of Digital Commons @ George Fox University. For more information, please contact [email protected]. Rivoli's Hummingbird Eugenes fulgens Order: CAPRIMULGIFORMES Family: TROCHILIDAE Version: 2.1 — Published June 27, 2018 Donald R. Powers Introduction Rivoli's Hummingbird was named in honor of the Duke of Rivoli when the species was described by René Lesson in 1829 (1). Even when it became known that William Swainson had written an earlier description of this species in 1827, the common name Rivoli's Hummingbird remained until the early 1980s, when it was changed to Magnificent Hummingbird. In 2017, however, the name was restored to Rivoli's Hummingbird when the American Ornithological Society officially recognized Eugenes fulgens as a distinct species from E. spectabilis, the Talamanca Hummingbird, of the highlands of Costa Rica and western Panama (2). See Systematics: Related Species. -

Vascular Plant and Vertebrate Inventory of Chiricahua National Monument
In Cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Chiricahua National Monument Open-File Report 2008-1023 U.S. Department of the Interior U.S. Geological Survey National Park Service This page left intentionally blank. In cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Chiricahua National Monument By Brian F. Powell, Cecilia A. Schmidt, William L. Halvorson, and Pamela Anning Open-File Report 2008-1023 U.S. Geological Survey Southwest Biological Science Center Sonoran Desert Research Station University of Arizona U.S. Department of the Interior School of Natural Resources U.S. Geological Survey 125 Biological Sciences East National Park Service Tucson, Arizona 85721 U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark Myers, Director U.S. Geological Survey, Reston, Virginia: 2008 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS-the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web:http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested Citation Powell, B.F., Schmidt, C.A., Halvorson, W.L., and Anning, Pamela, 2008, Vascular plant and vertebrate inventory of Chiricahua National Monument: U.S. Geological Survey Open-File Report 2008-1023, 104 p. [http://pubs.usgs.gov/of/2008/1023/]. Cover photo: Chiricahua National Monument. Photograph by National Park Service. Note: This report supersedes Schmidt et al. (2005). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. -

West Nile Virus Ecology in a Tropical Ecosystem in Guatemala
Am. J. Trop. Med. Hyg., 88(1), 2013, pp. 116–126 doi:10.4269/ajtmh.2012.12-0276 Copyright © 2013 by The American Society of Tropical Medicine and Hygiene West Nile Virus Ecology in a Tropical Ecosystem in Guatemala Maria E. Morales-Betoulle,† Nicholas Komar,*† Nicholas A. Panella, Danilo Alvarez, Marı´aR.Lo´pez, Jean-Luc Betoulle, Silvia M. Sosa, Marı´aL.Mu¨ ller, A. Marm Kilpatrick, Robert S. Lanciotti, Barbara W. Johnson, Ann M. Powers, Celia Cordo´ n-Rosales, and the Arbovirus Ecology Work Group‡ Center for Health Studies, Universidad del Valle de Guatemala, Guatemala; Centers for Disease Control and Prevention, Arbovirus Disease Branch, Fort Collins, Colorado; University of California, Santa Cruz, California; Fundacio´n Mario Dary, Guatemala City, Guatemala; and Fundacio´n para el Ecodesarrollo, Guatemala City, Guatemala Abstract. West Nile virus ecology has yet to be rigorously investigated in the Caribbean Basin. We identified a transmission focus in Puerto Barrios, Guatemala, and established systematic monitoring of avian abundance and infec- tion, seroconversions in domestic poultry, and viral infections in mosquitoes. West Nile virus transmission was detected annually between May and October from 2005 to 2008. High temperature and low rainfall enhanced the probability of chicken seroconversions, which occurred in both urban and rural sites. West Nile virus was isolated from Culex quinquefasciatus and to a lesser extent, from Culex mollis/Culex inflictus, but not from the most abundant Culex mosquito, Culex nigripalpus. A calculation that combined avian abundance, seroprevalence, and vertebrate reservoir competence suggested that great-tailed grackle (Quiscalus mexicanus) is the major amplifying host in this ecosystem. -

Sonoran Joint Venture Bird Conservation Plan Version 1.0
Sonoran Joint Venture Bird Conservation Plan Version 1.0 Sonoran Joint Venture 738 N. 5th Avenue, Suite 102 Tucson, AZ 85705 520-882-0047 (phone) 520-882-0037 (fax) www.sonoranjv.org May 2006 Sonoran Joint Venture Bird Conservation Plan Version 1.0 ____________________________________________________________________________________________ Acknowledgments We would like to thank all of the members of the Sonoran Joint Venture Technical Committee for their steadfast work at meetings and for reviews of this document. The following Technical Committee meetings were devoted in part or total to working on the Bird Conservation Plan: Tucson, June 11-12, 2004; Guaymas, October 19-20, 2004; Tucson, January 26-27, 2005; El Palmito, June 2-3, 2005, and Tucson, October 27-29, 2005. Another major contribution to the planning process was the completion of the first round of the northwest Mexico Species Assessment Process on May 10-14, 2004. Without the data contributed and generated by those participants we would not have been able to successfully assess and prioritize all bird species in the SJV area. Writing the Conservation Plan was truly a group effort of many people representing a variety of agencies, NGOs, and universities. Primary contributors are recognized at the beginning of each regional chapter in which they participated. The following agencies and organizations were involved in the plan: Arizona Game and Fish Department, Audubon Arizona, Centro de Investigación Cientifica y de Educación Superior de Ensenada (CICESE), Centro de Investigación de Alimentación y Desarrollo (CIAD), Comisión Nacional de Áreas Naturales Protegidas (CONANP), Instituto del Medio Ambiente y el Desarrollo (IMADES), PRBO Conservation Science, Pronatura Noroeste, Proyecto Corredor Colibrí, Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Sonoran Institute, The Hummingbird Monitoring Network, Tucson Audubon Society, U.S. -

Birding Tour
2020 JANUARY 4TH – 12TH, 2020 [Birding Tour] DOMINICAN REPUBLIC (HISPANIOLA) - 'Caribbean's Best Birding' - three endemic families of birds! Both evolution and geography has been 'kind' to the Island of Hispaniola with more 300 species of birds; THREE ENDEMIC FAMILES; six endemic genera; TWENTY-NINE ENDEMIC BIRDS; and 25 endemic subspecies. This is the Caribbean's most important center of avian variety... a 'must-see' birder's destination. Tour Style [B] $3,495/pp [Tour Filled] FEBRUARY 29TH – MARCH 14TH, 2020 [Birding Tour] OAXACA - ‘The Endemics of Mexico’s Southern Highlands, Isthmus of Tehuantepec, & Sierra Los Tuxtlas, Vera Cruz’ More than 50 of Mexico’s endemics occur in Oaxaca’s ‘Southern Highlands.’ This is a stand-alone destination that should be embraced by every birder interested in seeing the key species of Mexico. Tour Style [C] $3,795/pp [Tour Filled] RED WARBLER (Race 'rowleyi' is a Mexican Endemic regularly seen in Oaxaca State) MARCH 16TH – 29TH, 2020 [Birding Tour] CENTRAL MEXICO - ‘Finest Birding Route in North America’ – 450 SPECIES/60 MEXICAN ENDEMICS FOR THE ROUTE Route includes: [1] West Mexican Pacific slope, [2] Sierra Madre del Sur de Guerrero, [3] Balsas River drainage, and [4] Transvolcanic Belt. Tour Style [C] $3,895/pp [Tour Filled] 2020 APRIL 19TH – MAY 3RD, 2020 [Birding Tour]; BULGARIA ‘Classic Spring Birding’ The 2018 Tour recorded 232 species; expect up to 22 warblers, 9 tits, 10 woodpeckers, 5 owls, and 24 raptors, vultures, and falcons. Rare migrant birds mostly arrive from East Africa or West Asia. Food is delightful, travel is easy, accommodations are cozy, and dozens of times each day the scenic countryside shouts ‘photo’. -

The Tectonic Evolution of the Madrean Archipelago and Its Impact on the Geoecology of the Sky Islands
The Tectonic Evolution of the Madrean Archipelago and Its Impact on the Geoecology of the Sky Islands David Coblentz Earth and Environmental Sciences Division, Los Alamos National Laboratory, Los Alamos, NM Abstract—While the unique geographic location of the Sky Islands is well recognized as a primary factor for the elevated biodiversity of the region, its unique tectonic history is often overlooked. The mixing of tectonic environments is an important supplement to the mixing of flora and faunal regimes in contributing to the biodiversity of the Madrean Archipelago. The Sky Islands region is located near the actively deforming plate margin of the Western United States that has seen active and diverse tectonics spanning more than 300 million years, many aspects of which are preserved in the present-day geology. This tectonic history has played a fundamental role in the development and nature of the topography, bedrock geology, and soil distribution through the region that in turn are important factors for understanding the biodiversity. Consideration of the geologic and tectonic history of the Sky Islands also provides important insights into the “deep time” factors contributing to present-day biodiversity that fall outside the normal realm of human perception. in the North American Cordillera between the Sierra Madre Introduction Occidental and the Colorado Plateau – Southern Rocky The “Sky Island” region of the Madrean Archipelago (lo- Mountains (figure 1). This part of the Cordillera has been cre- cated between the northern Sierra Madre Occidental in Mexico ated by the interactions between the Pacific, North American, and the Colorado Plateau/Rocky Mountains in the Southwest- Farallon (now entirely subducted under North America) and ern United States) is an area of exceptional biodiversity and has Juan de Fuca plates and is rich in geology features, including become an important study area for geoecology, biology, and major plateaus (The Colorado Plateau), large elevated areas conservation management. -

Stories of the Sky Islands: Exhibit Development Resource Guide for Biology and Geology at Chiricahua National Monument and Coronado National Memorial
Stories of the Sky Islands: Exhibit Development Resource Guide for Biology and Geology at Chiricahua National Monument and Coronado National Memorial Prepared for the National Park Service under terms of Cooperative Ecosystems Studies Unit Agreement H1200-05-0003 Task Agreement J8680090020 Prepared by Adam M. Hudson,1 J. Jesse Minor,2,3 Erin E. Posthumus4 In cooperation with the Arizona State Museum The University of Arizona Tucson, AZ Beth Grindell, Principal Investigator May 17, 2013 1: Department of Geosciences, University of Arizona ([email protected]) 2: School of Geography and Development, University of Arizona ([email protected]) 3: Laboratory of Tree-Ring Research, University of Arizona 4: School of Natural Resources and the Environment, University of Arizona ([email protected]) Table of Contents Introduction ........................................................................................................................3 Beth Grindell, Ph.D. Ch. 1: Current research and information for exhibit development on the geology of Chiricahua National Monument and Coronado National Memorial, Southeast Arizona, USA..................................................................................................................................... 5 Adam M. Hudson, M.S. Section 1: Geologic Time and the Geologic Time Scale ..................................................5 Section 2: Plate Tectonic Evolution and Geologic History of Southeast Arizona .........11 Section 3: Park-specific Geologic History – Chiricahua -

United States Department of the Interior Fish and Wildlife Service Arizona Ecological Services Office 9828 N
United States Department of the Interior Fish and Wildlife Service Arizona Ecological Services Office 9828 N. 31st Avenue Ste C3 Phoenix, AZ 85051 Telephone: (602) 242-0210 Fax: (602) 242-2513 AESO/SE 22410-2011-F-0210 September 27, 2016 Ms. Laura Jo West, Forest Supervisor Coconino National Forest 1824 South Thompson Street Flagstaff, Arizona 8600 I RE: Rock Pits Project, Coconino and Kaibab National Forests Dear Ms. West: Thank you for your request for formal consultation with the U.S. Fish and Wildlife Service (FWS) pursuant to section 7 of the Endangered Species Act of 1973 (16 U.S.C. 1531-1544), as amended (Act). Your request and biological assessment (BA) were dated March 29, 2016, and received by us on April 4, 2016. This consultation concerns the potential effects of activities associated with the development and operation of rock pits on the Coconino and Kaibab National Forests in Coconino and Yavapai Counties, Arizona. The Forest Service has determined that the proposed action may affect, and is likely to adversely affect, the threatened Mexican spotted owl (Strix occidenta/is lucida) and its critical habitat. You have also requested our concurrence that the proposed action may affect, but is not likely to adversely affect the endangered California condor (Gymnogyps ca/ifornianus) outside of the lOj experimental nonessential population area, and "is not likely to jeopardize" the condor within the 1Oj experimental nonessential population area. We concur with your determinations. The basis for our concurrences is found in Appendix A. You also requested that we provide our technical assistance with respect to compliance with the Bald and Golden Eagle Protection Act (16 U.S.C. -

Spring 2009 RURAL LIVING in ARIZONA Volume 3, Number 2
ARIZONA COOPERATIVE E TENSION THE UNIVERSITY OF ARIZONA COLLEGE OF AGRICULTURE AND LIFE SCIENCES Backyards& Beyond Spring 2009 RURAL LIVING IN ARIZONA Volume 3, Number 2 Spring 2009 1 Common Name: Globemallow Scientific Name: Sphaeralcea spp. Globemallow is a common native wildflower found throughout most of Arizona. There are 16 species (and several varieties) occurring in the state, the majority of which are perennials. They are found between 1,000 and 6,000 feet in elevation and grow on a variety of soil types. Depending on the species, globemallows are either herbaceous or slightly woody at the base of the plant and grow between 2-3 feet in height (annual species may only grow to 6 inches). The leaves are three-lobed, and while the shape varies by species, they are similar enough to help identify the plant as a globemallow. The leaves have star-shaped hairs that give the foliage a gray-green color. Flower color Plant Susan Pater varies from apricot (the most common) to red, pink, lavender, pale yellow and white. Many of the globemallows flower in spring and again in summer. Another common name for globemallow is sore-eye poppy (mal de ojos in Spanish), from claims that the plant irritates the eyes. In southern California globemallows are known as plantas muy malas, translated to mean very bad plants. Ironically, the Pima Indian name for globemallow means a cure for sore eyes. The Hopi Indians used the plant for healing certain ailments and the stems as a type of chewing gum, and call the plant kopona. -

Variability and Distribution of the Golden-Headed Weevil Compsus Auricephalus (Say) (Curculionidae: Entiminae: Eustylini)
Biodiversity Data Journal 8: e55474 doi: 10.3897/BDJ.8.e55474 Single Taxon Treatment Variability and distribution of the golden-headed weevil Compsus auricephalus (Say) (Curculionidae: Entiminae: Eustylini) Jennifer C. Girón‡, M. Lourdes Chamorro§ ‡ Natural Science Research Laboratory, Museum of Texas Tech University, Lubbock, United States of America § Systematic Entomology Laboratory, ARS, USDA, c/o National Museum of Natural History, Smithsonian Institution, Washington, DC, United States of America Corresponding author: Jennifer C. Girón ([email protected]) Academic editor: Li Ren Received: 15 Jun 2020 | Accepted: 03 Jul 2020 | Published: 09 Jul 2020 Citation: Girón JC, Chamorro ML (2020) Variability and distribution of the golden-headed weevil Compsus auricephalus (Say) (Curculionidae: Entiminae: Eustylini). Biodiversity Data Journal 8: e55474. https://doi.org/10.3897/BDJ.8.e55474 Abstract Background The golden-headed weevil Compsus auricephalus is a native and fairly widespread species across the southern U.S.A. extending through Central America south to Panama. There are two recognised morphotypes of the species: the typical green form, with pink to cupreous head and part of the legs and the uniformly white to pale brown form. There are other Central and South American species of Compsus and related genera of similar appearance that make it challenging to provide accurate identifications of introduced species at ports of entry. New information Here, we re-describe the species, provide images of the habitus, miscellaneous morphological structures and male and female genitalia. We discuss the morphological variation of Compsus auricephalus across its distributional range, by revising and updating This is an open access article distributed under the terms of the CC0 Public Domain Dedication. -
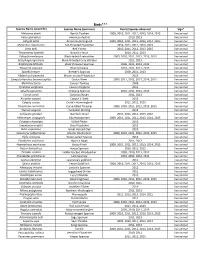
Flora and Fauna List.Xlsx
Birds*** Species Name (scientific) Species Name (common) Year(s) Species observed Sign* Melozone aberti Abert's Towhee 2009, 2010, 2011, 2012, 2013, 2014, 2015 live animal Falco sparverius American Kestrel 2010, 2013 live animal Calypte anna Anna's Hummingbird 2009, 2010, 2011, 2012, 2013, 2014, 2015 live animal Myiarchus cinerascens Ash‐throated Flycatcher 2010, 2011, 2012, 2013, 2015 live animal Vireo bellii Bell's Vireo 2010, 2011, 2012, 2013, 2015 live animal Thryomanes bewickii Bewick's Wren 2010, 2011, 2013 live animal Polioptila melanura Black‐tailed Gnatcatcher 2009, 2010, 2011, 2012, 2013, 2015 live animal Setophaga nigrescens Black‐throated Gray Warbler 2011, 2013 live animal Amphispiza bilineata Black‐throated Sparrow 2009, 2011, 2012, 2013 live animal Passerina caerulea Blue Grosbeak 2010, 2011, 2012, 2013 live animal Spizella breweri Brewer's Sparrow 2009, 2011, 2013 live animal Myiarchus tryannulus Brown‐crested Flycatcher 2013 live animal Campylorhynchus brunneicapillus Cactus Wren 2009, 2011, 2012, 2013, 2014, 2015 live animal Melozone fusca Canyon Towhee 2009 live animal Tyrannus vociferans Cassin's Kingbird 2011 live animal Spizella passerina Chipping Sparrow 2010, 2011, 2012, 2013 live animal Corvus corax Common Raven 2011, 2013 live animal Accipiter cooperii Cooper's Hawk 2013 live animal Calypte costae Costa's Hummingbird 2011, 2012, 2013 live animal Toxostoma curvirostre Curve‐billed Thrasher 2009, 2010, 2011, 2012, 2013, 2015 live animal Sturnus vulgaris European Starling 2012 live animal Callipepla gambelii Gambel's