Variability and Distribution of the Golden-Headed Weevil Compsus Auricephalus (Say) (Curculionidae: Entiminae: Eustylini)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bill Shape As a Generic Character in the Cardinals
BILL SHAPE AS A GENERIC CHARACTER IN THE CARDINALS WALTER J. BOCK ANY genera in birds and other animal groups have been based essentially M upon a single character. This character may be a single morphological feature, such as the presence or absence of the hallux, or it may be a complex of characters which are all closely correlated functionally, such as the bones, muscles, and ligaments of the jaw apparatus. The validity of many of these genera has been questioned in recent years with the general acceptance of the polytypic species concept and the increasing acknowledgment of the grouping service at low taxonomic levels provided by the genus. An example is the North American passerine genus Pyrrhuloxia, which is distinguished from Richmondena essentially on the basis of bill shape. The overall similarity of Pyrrhuloxia sinuata to the two species of Richmondena in morphology and in general life history (Gould, 1961) has led several recent authors to synony- mize Richmondena with Pyrrhuloxia. Other workers have maintained the validity of the generic separation, basing their decision largely on the dif- ference in bill shape. The object of this paper is to ascertain the importance of this difference as a taxonomic character and whether the difference if con- firmed is of generic significance. THE JAW APPARATUS Ridgway (19013624-625) described the bill of P. sinuata (see Figs. 1 and 2) as follows: “Bill very short, thick and deep, with culmen strongly convex and maxillary tomium deeply and angularly incised a little posterior to the middle -

SUSCEPTIBILIDAD DE Compsus N
SUSCEPTIBILIDAD DE Compsus n. sp. A Beauveria bassiana (Bals.) Vuill y Metarhizium anisopliae (Metsch.) Sorokin SUSCEPTIBILITY OF Compsus n. sp. to Beauveria bassiana (Bals.) Vuill and Metarhizium anisopliae (Metsch.) Sorokin SILVIA CONSTANZA OROZCO PUERTA Ingeniera Agropecuaria Tesis de Grado presentada como requisito parcial para optar el titulo en Magíster en Ciencias – Entomología DIRECTOR Juan Humberto Guarín Molina, Ph.D. Entomología UNIVERSIDAD NACIONAL DE COLOMBIA SEDE DE MEDELLÍN FACULTAD DE CIENCIAS 2011 AGRADECIMIENTOS La autora expresa sus agradecimientos a: CORPOICA, Citricauca y MADR. Elizabeth Meneses de CORPOICA. Guillermo Correa, Docente de la Universidad Nacional de Colombia Sede Medellín, por la asesoría estadística. Equipo de trabajo en Tulio Ospina: Gabriel Rendón, Tatiana Restrepo y Verónica Álvarez. Juan Humberto Guarín. A mi familia, amigos y compañeros que siempre me brindaron apoyo incondicional. A los profesores Rodrigo Vergara y Francisco Yepes por ayudarme a mejorar mi trabajo con sus recomendaciones. ii CONTENIDO Pág. LISTA DE TABLAS ............................................................................................... IV LISTA DE FIGURAS .............................................................................................. V LISTA DE ANEXOS ............................................................................................. VII RESUMEN ............................................................................................................... 1 ABSTRACT ............................................................................................................ -

Arthropod Pests of Citrus Roots
lds. r at ex ual to ap ila red t is een vi Clayton W. McCoy fa University of Florida ks Citrus Res ea rch and Educati on Center, Lake Alfred )0 Ily I'::y les Ill up 10 Arthropod Pests of Citrus Roots 'ul r-J!l 'Ie '](1 cc The major arthropods that are injurious to plant roots are Geographical Distribution members of the classes Insecta and Acari (mites). Two-thi rds of these pests are members of the order Coleoptera (beetles), Citrus root weevi ls are predominantly trop ical ; however, a which as larvae cause serious economic loss in a wide range few temperate species are important pests in the United States, of plan t hosts. Generally, the larvae hatch from eggs laid by Chile. Argentina. Australia. and New Zealand (Table 14.1). adults on plan ts or in the soil and complete part of their life The northern blue-green citrus root weevil, Pachnaeus opalus; cycle chewing on plant roots, and in many cases as adults the Fuller rose beetle, Asynonychus godmani: and related spe they feed on the foli age of the same or other host plan ts. A cies in the genus Pantomorus are found in temperate areas. Ap number of arthropods inhabit the rhizosphere of citrus trees. proximately 150 species have been recorded in the Caribbean some as unique syrnbionts, but few arc injurious to the roots. region, including Florida. Central America, and South America, Only citrus root weevils. termi tes. and ants. in descending or feeding as larvae on the roots of all species of the genus Citrus. -

Coleoptera) (Excluding Anthribidae
A FAUNAL SURVEY AND ZOOGEOGRAPHIC ANALYSIS OF THE CURCULIONOIDEA (COLEOPTERA) (EXCLUDING ANTHRIBIDAE, PLATPODINAE. AND SCOLYTINAE) OF THE LOWER RIO GRANDE VALLEY OF TEXAS A Thesis TAMI ANNE CARLOW Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 1997 Major Subject; Entomology A FAUNAL SURVEY AND ZOOGEOGRAPHIC ANALYSIS OF THE CURCVLIONOIDEA (COLEOPTERA) (EXCLUDING ANTHRIBIDAE, PLATYPODINAE. AND SCOLYTINAE) OF THE LOWER RIO GRANDE VALLEY OF TEXAS A Thesis by TAMI ANNE CARLOW Submitted to Texas AgcM University in partial fulltllment of the requirements for the degree of MASTER OF SCIENCE Approved as to style and content by: Horace R. Burke (Chair of Committee) James B. Woolley ay, Frisbie (Member) (Head of Department) Gilbert L. Schroeter (Member) August 1997 Major Subject: Entomology A Faunal Survey and Zoogeographic Analysis of the Curculionoidea (Coleoptera) (Excluding Anthribidae, Platypodinae, and Scolytinae) of the Lower Rio Grande Valley of Texas. (August 1997) Tami Anne Carlow. B.S. , Cornell University Chair of Advisory Committee: Dr. Horace R. Burke An annotated list of the Curculionoidea (Coleoptem) (excluding Anthribidae, Platypodinae, and Scolytinae) is presented for the Lower Rio Grande Valley (LRGV) of Texas. The list includes species that occur in Cameron, Hidalgo, Starr, and Wigacy counties. Each of the 23S species in 97 genera is tteated according to its geographical range. Lower Rio Grande distribution, seasonal activity, plant associations, and biology. The taxonomic atTangement follows O' Brien &, Wibmer (I og2). A table of the species occuning in patxicular areas of the Lower Rio Grande Valley, such as the Boca Chica Beach area, the Sabal Palm Grove Sanctuary, Bentsen-Rio Grande State Park, and the Falcon Dam area is included. -

The Beetle Fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and Distribution
INSECTA MUNDI, Vol. 20, No. 3-4, September-December, 2006 165 The beetle fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and distribution Stewart B. Peck Department of Biology, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6, Canada stewart_peck@carleton. ca Abstract. The beetle fauna of the island of Dominica is summarized. It is presently known to contain 269 genera, and 361 species (in 42 families), of which 347 are named at a species level. Of these, 62 species are endemic to the island. The other naturally occurring species number 262, and another 23 species are of such wide distribution that they have probably been accidentally introduced and distributed, at least in part, by human activities. Undoubtedly, the actual numbers of species on Dominica are many times higher than now reported. This highlights the poor level of knowledge of the beetles of Dominica and the Lesser Antilles in general. Of the species known to occur elsewhere, the largest numbers are shared with neighboring Guadeloupe (201), and then with South America (126), Puerto Rico (113), Cuba (107), and Mexico-Central America (108). The Antillean island chain probably represents the main avenue of natural overwater dispersal via intermediate stepping-stone islands. The distributional patterns of the species shared with Dominica and elsewhere in the Caribbean suggest stages in a dynamic taxon cycle of species origin, range expansion, distribution contraction, and re-speciation. Introduction windward (eastern) side (with an average of 250 mm of rain annually). Rainfall is heavy and varies season- The islands of the West Indies are increasingly ally, with the dry season from mid-January to mid- recognized as a hotspot for species biodiversity June and the rainy season from mid-June to mid- (Myers et al. -

Lista De Especies De Curculionoidea Depositadas En La Colecci.N De
Acta Zool. Mex. (n.s.) 87: 147-165 (2002) LISTA DE LAS ESPECIES DE CURCULIONOIDEA (INSECTA: COLEOPTERA) DEPOSITADAS EN LA COLECCIÓN DEL MUSEO DE ZOOLOGÍA "ALFONSO L. HERRERA", FACULTAD DE CIENCIAS, UNAM (MZFC) Juan J. MORRONE1, Raúl MUÑIZ2, Julieta ASIAIN3 y Juan MÁRQUEZ1,3 1 Museo de Zoología, Departamento de Biología Evolutiva, Facultad de Ciencias, UNAM, Apdo. postal 70-399, CP 04510 México D.F., MÉXICO 2 Lago Cuitzeo # 144, CP 11320. México, D. F. MÉXICO 3 Laboratorio Especializado de Morfofisiología Animal, Facultad de Ciencias, UNAM, Apdo. postal 70-399, CP 04510 México D.F., MÉXICO RESUMEN La colección del Museo de Zoología "Alfonso L. Herrera" incluye 1,148 especímenes de Curculionoidea, que pertenecen a 397 especies, 217 géneros y 14 familias. La familia mejor representada es Curculionidae, con 342 especies, seguida de Dryophthoridae (12), Erirhinidae (8), Belidae (7), Oxycorynidae (6), Brentidae (4), Nemonychidae (4), Rhynchitidae (4), Anthribidae (3), Apionidae (2), Brachyceridae (2), Attelabidae (1), Ithyceridae (1) y Raymondionymidae (1). Muchos de los especímenes provienen de otros países (Argentina, Chile, Brasil y E.U.A., entre otros). La mayoría de los ejemplares mexicanos son de los estados de Hidalgo (44 especies), Morelos (13), Nayarit (12) y Veracruz (12). Palabras Clave: Coleoptera, Curculionoidea, Curculionidae, colección. ABSTRACT The collection of the Museo de Zoología "Alfonso L. Herrera" includes 1,148 specimens of Curculionoidea, which belong to 397 species, 217 genera, and 14 families. The best represented family is Curculionidae, with 342 species, followed by Dryophthoridae (12), Erirhinidae (8), Belidae (7), Oxycorynidae (6), Brentidae (4), Nemonychidae (4), Rhynchitidae (4), Anthribidae (3), Apionidae (2), Brachyceridae (2), Attelabidae (1), Ithyceridae (1), and Raymondionymidae (1). -

Fossil History of Curculionoidea (Coleoptera) from the Paleogene
geosciences Review Fossil History of Curculionoidea (Coleoptera) from the Paleogene Andrei A. Legalov 1,2 1 Institute of Systematics and Ecology of Animals, Siberian Branch, Russian Academy of Sciences, Ulitsa Frunze, 11, 630091 Novosibirsk, Novosibirsk Oblast, Russia; [email protected]; Tel.: +7-9139471413 2 Biological Institute, Tomsk State University, Lenin Ave, 36, 634050 Tomsk, Tomsk Oblast, Russia Received: 23 June 2020; Accepted: 4 September 2020; Published: 6 September 2020 Abstract: Currently, some 564 species of Curculionoidea from nine families (Nemonychidae—4, Anthribidae—33, Ithyceridae—3, Belidae—9, Rhynchitidae—41, Attelabidae—3, Brentidae—47, Curculionidae—384, Platypodidae—2, Scolytidae—37) are known from the Paleogene. Twenty-seven species are found in the Paleocene, 442 in the Eocene and 94 in the Oligocene. The greatest diversity of Curculionoidea is described from the Eocene of Europe and North America. The richest faunas are known from Eocene localities, Florissant (177 species), Baltic amber (124 species) and Green River formation (75 species). The family Curculionidae dominates in all Paleogene localities. Weevil species associated with herbaceous vegetation are present in most localities since the middle Paleocene. A list of Curculionoidea species and their distribution by location is presented. Keywords: Coleoptera; Curculionoidea; fossil weevil; faunal structure; Paleocene; Eocene; Oligocene 1. Introduction Research into the biodiversity of the past is very important for understanding the development of life on our planet. Insects are one of the Main components of both extinct and recent ecosystems. Coleoptera occupied a special place in the terrestrial animal biotas of the Mesozoic and Cenozoics, as they are characterized by not only great diversity but also by their ecological specialization. -

Biologic Notes on Certain Iowa Insects
Proceedings of the Iowa Academy of Science Volume 3 Annual Issue Article 39 1895 Biologic Notes on Certain Iowa Insects Herbert Osborn C. W. Mally Let us know how access to this document benefits ouy Copyright ©1895 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Osborn, Herbert and Mally, C. W. (1895) "Biologic Notes on Certain Iowa Insects," Proceedings of the Iowa Academy of Science, 3(1), 203-213. Available at: https://scholarworks.uni.edu/pias/vol3/iss1/39 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Osborn and Mally: Biologic Notes on Certain Iowa Insects IOWA ACADEMY OF SCIENCES. 203 MELAMPSALTA PARVULA SAY. This interesting little species has been taken once at Ames and this is, so far as I know, the only record of its occurrence in the state. It is a more southern form, being credited to the southern states as far north as southern Illinois and central Kansas. Very likely it may be found occasionally in the south ern part of the state when collectors become more plentiful. Any addition to these records will be gratefully received and duly credited in future records. BIOLOGIC NOTES ON CERTAIN IOWA INSECTS. HERBERT OSBORN AND C. W. MALLY. The following notes are extracted from Bulletin 32 of the Iowa Experiment Station, and embrace such portions of work upon certain injurious insects as have a biologic interest. -

Spring 2009 RURAL LIVING in ARIZONA Volume 3, Number 2
ARIZONA COOPERATIVE E TENSION THE UNIVERSITY OF ARIZONA COLLEGE OF AGRICULTURE AND LIFE SCIENCES Backyards& Beyond Spring 2009 RURAL LIVING IN ARIZONA Volume 3, Number 2 Spring 2009 1 Common Name: Globemallow Scientific Name: Sphaeralcea spp. Globemallow is a common native wildflower found throughout most of Arizona. There are 16 species (and several varieties) occurring in the state, the majority of which are perennials. They are found between 1,000 and 6,000 feet in elevation and grow on a variety of soil types. Depending on the species, globemallows are either herbaceous or slightly woody at the base of the plant and grow between 2-3 feet in height (annual species may only grow to 6 inches). The leaves are three-lobed, and while the shape varies by species, they are similar enough to help identify the plant as a globemallow. The leaves have star-shaped hairs that give the foliage a gray-green color. Flower color Plant Susan Pater varies from apricot (the most common) to red, pink, lavender, pale yellow and white. Many of the globemallows flower in spring and again in summer. Another common name for globemallow is sore-eye poppy (mal de ojos in Spanish), from claims that the plant irritates the eyes. In southern California globemallows are known as plantas muy malas, translated to mean very bad plants. Ironically, the Pima Indian name for globemallow means a cure for sore eyes. The Hopi Indians used the plant for healing certain ailments and the stems as a type of chewing gum, and call the plant kopona. -

Weevils) of the George Washington Memorial Parkway, Virginia
September 2020 The Maryland Entomologist Volume 7, Number 4 The Maryland Entomologist 7(4):43–62 The Curculionoidea (Weevils) of the George Washington Memorial Parkway, Virginia Brent W. Steury1*, Robert S. Anderson2, and Arthur V. Evans3 1U.S. National Park Service, 700 George Washington Memorial Parkway, Turkey Run Park Headquarters, McLean, Virginia 22101; [email protected] *Corresponding author 2The Beaty Centre for Species Discovery, Research and Collection Division, Canadian Museum of Nature, PO Box 3443, Station D, Ottawa, ON. K1P 6P4, CANADA;[email protected] 3Department of Recent Invertebrates, Virginia Museum of Natural History, 21 Starling Avenue, Martinsville, Virginia 24112; [email protected] ABSTRACT: One-hundred thirty-five taxa (130 identified to species), in at least 97 genera, of weevils (superfamily Curculionoidea) were documented during a 21-year field survey (1998–2018) of the George Washington Memorial Parkway national park site that spans parts of Fairfax and Arlington Counties in Virginia. Twenty-three species documented from the parkway are first records for the state. Of the nine capture methods used during the survey, Malaise traps were the most successful. Periods of adult activity, based on dates of capture, are given for each species. Relative abundance is noted for each species based on the number of captures. Sixteen species adventive to North America are documented from the parkway, including three species documented for the first time in the state. Range extensions are documented for two species. Images of five species new to Virginia are provided. Keywords: beetles, biodiversity, Malaise traps, national parks, new state records, Potomac Gorge. INTRODUCTION This study provides a preliminary list of the weevils of the superfamily Curculionoidea within the George Washington Memorial Parkway (GWMP) national park site in northern Virginia. -
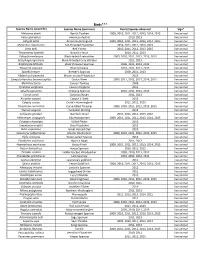
Flora and Fauna List.Xlsx
Birds*** Species Name (scientific) Species Name (common) Year(s) Species observed Sign* Melozone aberti Abert's Towhee 2009, 2010, 2011, 2012, 2013, 2014, 2015 live animal Falco sparverius American Kestrel 2010, 2013 live animal Calypte anna Anna's Hummingbird 2009, 2010, 2011, 2012, 2013, 2014, 2015 live animal Myiarchus cinerascens Ash‐throated Flycatcher 2010, 2011, 2012, 2013, 2015 live animal Vireo bellii Bell's Vireo 2010, 2011, 2012, 2013, 2015 live animal Thryomanes bewickii Bewick's Wren 2010, 2011, 2013 live animal Polioptila melanura Black‐tailed Gnatcatcher 2009, 2010, 2011, 2012, 2013, 2015 live animal Setophaga nigrescens Black‐throated Gray Warbler 2011, 2013 live animal Amphispiza bilineata Black‐throated Sparrow 2009, 2011, 2012, 2013 live animal Passerina caerulea Blue Grosbeak 2010, 2011, 2012, 2013 live animal Spizella breweri Brewer's Sparrow 2009, 2011, 2013 live animal Myiarchus tryannulus Brown‐crested Flycatcher 2013 live animal Campylorhynchus brunneicapillus Cactus Wren 2009, 2011, 2012, 2013, 2014, 2015 live animal Melozone fusca Canyon Towhee 2009 live animal Tyrannus vociferans Cassin's Kingbird 2011 live animal Spizella passerina Chipping Sparrow 2010, 2011, 2012, 2013 live animal Corvus corax Common Raven 2011, 2013 live animal Accipiter cooperii Cooper's Hawk 2013 live animal Calypte costae Costa's Hummingbird 2011, 2012, 2013 live animal Toxostoma curvirostre Curve‐billed Thrasher 2009, 2010, 2011, 2012, 2013, 2015 live animal Sturnus vulgaris European Starling 2012 live animal Callipepla gambelii Gambel's -

Section IV – Guideline for the Texas Priority Species List
Section IV – Guideline for the Texas Priority Species List Associated Tables The Texas Priority Species List……………..733 Introduction For many years the management and conservation of wildlife species has focused on the individual animal or population of interest. Many times, directing research and conservation plans toward individual species also benefits incidental species; sometimes entire ecosystems. Unfortunately, there are times when highly focused research and conservation of particular species can also harm peripheral species and their habitats. Management that is focused on entire habitats or communities would decrease the possibility of harming those incidental species or their habitats. A holistic management approach would potentially allow species within a community to take care of themselves (Savory 1988); however, the study of particular species of concern is still necessary due to the smaller scale at which individuals are studied. Until we understand all of the parts that make up the whole can we then focus more on the habitat management approach to conservation. Species Conservation In terms of species diversity, Texas is considered the second most diverse state in the Union. Texas has the highest number of bird and reptile taxon and is second in number of plants and mammals in the United States (NatureServe 2002). There have been over 600 species of bird that have been identified within the borders of Texas and 184 known species of mammal, including marine species that inhabit Texas’ coastal waters (Schmidly 2004). It is estimated that approximately 29,000 species of insect in Texas take up residence in every conceivable habitat, including rocky outcroppings, pitcher plant bogs, and on individual species of plants (Riley in publication).