Volcanic Controls on the Microbial Habitability of Mars-Analogue Hydrothermal Environments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bio-Preservation Potential of Sediment in Eberswalde Crater, Mars
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship Fall 2020 Bio-preservation Potential of Sediment in Eberswalde crater, Mars Cory Hughes Western Washington University, [email protected] Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Geology Commons Recommended Citation Hughes, Cory, "Bio-preservation Potential of Sediment in Eberswalde crater, Mars" (2020). WWU Graduate School Collection. 992. https://cedar.wwu.edu/wwuet/992 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. Bio-preservation Potential of Sediment in Eberswalde crater, Mars By Cory M. Hughes Accepted in Partial Completion of the Requirements for the Degree Master of Science ADVISORY COMMITTEE Dr. Melissa Rice, Chair Dr. Charles Barnhart Dr. Brady Foreman Dr. Allison Pfeiffer GRADUATE SCHOOL David L. Patrick, Dean Master’s Thesis In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permissions from the owner of any third party copyrighted material included in these files. -
Cambridge University Press 978-1-107-03629-1 — the Atlas of Mars Kenneth S
Cambridge University Press 978-1-107-03629-1 — The Atlas of Mars Kenneth S. Coles, Kenneth L. Tanaka, Philip R. Christensen Index More Information Index Note: page numbers in italic indicates figures or tables Acheron Fossae 76, 76–77 cuesta 167, 169 Hadriacus Cavi 183 orbit 1 Acidalia Mensa 86, 87 Curiosity 9, 32, 62, 195 Hadriacus Palus 183, 184–185 surface gravity 1, 13 aeolian, See wind; dunes Cyane Catena 82 Hecates Tholus 102, 103 Mars 3 spacecraft 6, 201–202 Aeolis Dorsa 197 Hellas 30, 30, 53 Mars Atmosphere and Volatile Evolution (MAVEN) 9 Aeolis Mons, See Mount Sharp Dao Vallis 227 Hellas Montes 225 Mars Chart 1 Alba Mons 80, 81 datum (zero elevation) 2 Hellas Planitia 220, 220, 226, 227 Mars Exploration Rovers (MER), See Spirit, Opportunity albedo 4, 5,6,10, 56, 139 deformation 220, See also contraction, extension, faults, hematite 61, 130, 173 Mars Express 9 alluvial deposits 62, 195, 197, See also fluvial deposits grabens spherules 61,61 Mars Global Surveyor (MGS) 9 Amazonian Period, history of 50–51, 59 Deimos 62, 246, 246 Henry crater 135, 135 Mars Odyssey (MO) 9 Amenthes Planum 143, 143 deltas 174, 175, 195 Herschel crater 188, 189 Mars Orbiter Mission (MOM) 9 Apollinaris Mons 195, 195 dikes, igneous 82, 105, 155 Hesperia Planum 188–189 Mars Pathfinder 9, 31, 36, 60,60 Aram Chaos 130, 131 domical mound 135, 182, 195 Hesperian Period, history of 50, 188 Mars Reconnaissance Orbiter (MRO) 9 Ares Vallis 129, 130 Dorsa Argentea 239, 240 Huygens crater 183, 185 massif 182, 224 Argyre Planitia 213 dunes 56, 57,69–70, 71, 168, 185, -

Briony H. N. Horgan
December 2019 Briony H. N. Horgan 550 Stadium Mall Drive, West Lafayette, IN 47907 Assistant Professor [email protected] Department of Earth, Atmospheric, and Planetary Sciences (503) 703-8473 & School of Aeronautics and Astronautics Purdue University Education and Appointments 2014-present Assistant Professor, Purdue University 2013 Faculty Research Associate, Arizona State University 2010-2012 Exploration Postdoctoral Fellow, Arizona State University Advisor: Prof. Phil Christensen 2005-2010 Ph.D., Cornell University, Astronomy and Space Sciences Advisor: Prof. Jim Bell 2001-2005 B.S., Oregon State University, Physics, summa cum laude Fields of Expertise • Surface geology, mineralogy, rover studies, and remote sensing of the terrestrial planets • Visible, near-infrared, and thermal-infrared spectroscopy • Mapping and analysis of large hyperspectral datasets • Planetary analog field studies of weathering, aeolian, volcanic, and glacial processes NASA Mission Experience 2016-present Participating Scientist, Mars Science Laboratory Mission 2015-present Co-I, Mastcam-Z imaging investigation, Mars2020 Mission 2008-2014 Science Team, THEMIS, Mars Odyssey Mission Support: Current and Past 2021-2024 Co-Investigator, NASA Solar Systems Working Program Between a rock and a frozen place: Cold-based glacial chemical alteration of volcanic bedrock as an analog for Mars (PI: Dr. Alicia Rutledge) 2020-2021 Co-Investigator, NASA Planetary Mission Concepts Studies Mars Orbiter for Resources, Ice, and Environment (PI: Prof. Wendy Calvin) 2020-2023 Co-Investigator, NASA Mars2020 Mission Phase E Mastcam-Z: A Geologic, Stereoscopic, and Multispectral Investigation for the NASA Mars 2020 Rover Mission (PI: Prof. Jim Bell) 2020-2023 Co-Investigator, NASA Lunar Data Analysis Program Investigating Explosive Volcanic Deposits in the Montes Apenninus area using Moon Mineralogy Mapper Data (PI: Dr. -

The Dorsa Argentea Formation: Synthesis of Glacial Features and History of Late Noachian-Early Hesperian Martian Climate Change
45th Lunar and Planetary Science Conference (2014) 1477.pdf THE DORSA ARGENTEA FORMATION: SYNTHESIS OF GLACIAL FEATURES AND HISTORY OF LATE NOACHIAN-EARLY HESPERIAN MARTIAN CLIMATE CHANGE. K. E. Scanlon and J. W. Head III, Department of Geological Sciences, Brown University, Providence, RI, USA. <[email protected]> Introduction: The Noachian and Hesperian-aged ge- Cavi Angusti and Argentea Planum have been inter- omorphological units mapped [1-2] as the Dorsa Ar- preted as the remnants of a proglacial lake [3, 21]. gentea Formation (DAF) and the Hesperian-Noachian undivided unit cover a combined area of ~1.5 · 106 km2 [3] surrounding and offset from the south pole of Mars. The units are characterized by sinuous and braided ridges, including the Dorsa Argentea for which the formation is named [e.g. 4-10], several regions of pit- ted terrain, the Cavi Angusti and Sisyphi Cavi [e.g. 11- 13]; and steep-sided mountains, the Sisyphi Montes [e.g. 14]. A radar reflector closely correlated with the outline of the deposit is consistent with high concentra- tions of volatiles in and underlying the deposit [15]. In earlier work based on Mariner and Viking im- ages, researchers proposed volcanic, tectonic, inverted stream, aeolian, and clastic dike origins for the Dorsa Argentea and other sinuous ridges (e.g., [1, 5]; see re- view in [3]) and widely agreed on an aeolian origin for the cavi [e.g. 11, 12]. Howard [4] was the first to pro- Fig. 1. Features interpreted to be of glacial origin in the Dorsa Ar- pose a glacial origin for the deposit, wherein the sinu- gentea formation include cavi interpreted as melt-out terrains [e.g. -

Sisyphi Montes and Southwest Hellas Paterae: Possible Impact, Cryotectonic, Volcanic, and Mantle Tectonic Processes Along Hellas Basin Rings
Fourth Mars Polar Science Conference (2006) 8066.pdf SISYPHI MONTES AND SOUTHWEST HELLAS PATERAE: POSSIBLE IMPACT, CRYOTECTONIC, VOLCANIC, AND MANTLE TECTONIC PROCESSES ALONG HELLAS BASIN RINGS. J.A.P. Rodrigu- ez1 and K.L. Tanaka2 ; 1PSI, Tucson, AZ 85719; 2Astrogeology Team, U.S. Geological Survey, Flagstaff, AZ 86001. Introduction. Sisyphi Montes form a chain of A Sisyphi-Malea tectonomagmatic complex? The prominent circular mountains within Sisyphi Planum (57 paterae of Malea Planum have a topographic and geo- to 75ºS, -12 to 34ºE) surrounded by circum-polar and logic form similar to some Venusian coronae [e.g., 3]. circum-Hellas highlands on Mars [1]. A variety of im- They consist of annular topographic highs hundreds of pact and volcanic origins have been considered for them, kilometers across surrounding interior depressions and as reviewed in [2]. Based on the flat tops, summit craters, thought to be made up of volcanic flows and deposits and associated channels that many of them possess, deformed by compressional structures manifested by Ghatan and Head [2] interpreted them to be subglacial concentric and radiating wrinkle ridges. Although volcanoes. Here, we identify additional geologic con- Sisyphi Planum does not include paterae forms, its resur- trols and various alternative processes that may have led facing may be related to early, intense magmatic activity. to their formation. For example, this part of the margin of Hellas basin Regional geologic setting. Our regional analysis re- appears to be deeply eroded, perhaps due to intense veals that nearly all major volcanic and tectonic features intrusive activity during the Early to Middle Noachian within the Hellas region occur within 100 km of possible [4]. -

South Circumpolar Ice Sheet on Mars: Regional Drainage of Meltwater Beneath the Hesperian-Aged Dorsa Argentea Formation
Sixth International Conference on Mars (2003) 3034.pdf SOUTH CIRCUMPOLAR ICE SHEET ON MARS: REGIONAL DRAINAGE OF MELTWATER BENEATH THE HESPERIAN-AGED DORSA ARGENTEA FORMATION. Gil J. Ghatan, and James W. Head, Department of Geological. Sciences, Brown University, Providence, RI 02912, USA, Gil_Ghatan@Brown. Introduction and Background: Additional work has been done since that of Head and The south polar region of Mars is currently the site of an Pratt [3], which further supports the ice sheet hypothesis. expansive ice cap, covering an area of approximately 1.42 x Ghatan and Head [5] performed a detailed investigation of a 106 km2, and composed of two mapped units: Api, a residual, series of massifs (Sisyphi Montes) located within the 0W-lobe high albedo unit; and Apl, a lower albedo unit that underlies of the DAF and interpreted the mountains as subglacial volca- Api, and in places displays bright and dark layering [1]. Age noes that erupted into a thick Hesperian-aged ice sheet, possi- constraints on the present polar cap obtained from crater reten- bly resulting in local meltback. A study by Milkovich et al. [6] tion have attributed a surface age for the deposits to the up- provides evidence for extensive, lateral migration of meltwater permost Amazonian [1, 2]. away from the eastern margins of the 0W-lobe, down into the Surrounding the present polar cap, are a series of deposits, Prometheus basin. Ghatan et al. [7] have examined the Cavi which together compose the Dorsa Argentea Formation. Angusti area of the 70W-lobe in detail, and have interpreted Originally mapped as two units (an upper and lower member) the collection of irregularly shaped, steep-sided basins as hav- [1], the DAF is asymmetrically distributed about the current ing formed from volatile loss due to a combination of heating polar deposits, forming two main lobes, one centered about associated with intrusive and extrusive magmatic activity. -
Before You Continue
RECENT GEOLOGIC MAPPING RESULTS FOR THE POLAR REGIONS OF MARS. K.L. Tanaka1 and E.J. Kolb2, 1Astrogeology Team, U.S. Geological Survey, Flagstaff, AZ 86001, [email protected], 2Google, Inc., Mountain View, CA 94043, [email protected]. Introduction: The polar regions of Mars include the steep scarps exposed on the margins of Abalos Mensa, densest data coverage for the planet because of the polar from Boreum and Tenuis Cavi at the head of Chasma orbits of MGS, ODY, and MEX. Because the geology of Boreale, and from reentrants of Olympia Cavi into the polar plateaus has been among the most dynamic on Planum Boreum. The bases of the scarps include dark, the planet in recent geologic time, the data enable the cross-bedded bright and dark layered material mapped most detailed and complex geologic investigations of any as the Planum Boreum cavi unit (ABbc) [7, 10]. Some regions on Mars, superseding previous, even recent, map- of the dunes of Olympia Undae are embayed by the ping efforts [e.g., 1-3]. Geologic mapping at regional and young mid-latitude mantle [11] and the youngest polar local scales is revealing that the stratigraphy and modifi- layered deposits (Planum Boreum unit 2). cational histories of polar materials by various processes The Planum Boreum cavi unit grades upwards into are highly complex at both poles. Here, we describe some Planum Boreum 1 unit (ABb1), which forms the major- of our recent results in polar geologic mapping and how ity of what are commonly referred to as “polar layered they address the geologic processes involved and implica- deposits” (however, other polar deposits are also lay- tions for polar climate history. -

Volcanism and Tectonism Across the Inner Solar System: an Overview
Downloaded from http://sp.lyellcollection.org/ by guest on September 23, 2021 Volcanism and tectonism across the inner solar system: an overview T. PLATZ1,2*, P. K. BYRNE3,4, M. MASSIRONI5 & H. HIESINGER6 1Planetary Science Institute, 1700 East Fort Lowell Road, Tucson, AZ 85719-2395, USA 2Freie Universita¨t Berlin, Institute of Geological Sciences, Planetary Sciences & Remote Sensing, Malteserstrasse 74-100, 12249 Berlin, Germany 3Lunar and Planetary Institute, Universities Space Research Association, 3600 Bay Area Boulevard, Houston, TX 77058, USA 4Department of Terrestrial Magnetism, Carnegie Institution of Washington, 5241 Broad Branch Road NW, Washington, DC 20015-1305, USA 5Dipartimento di Geoscienze, Universita’ degli Studi di Padova, via G. Gradenigo 6, 35131 Padova, Italy 6Institut fu¨r Planetologie, Westfa¨lische Wilhelms-Universita¨tMu¨nster, Wilhelm-Klemm-Strasse 10, 48149 Mu¨nster, Germany *Corresponding author (e-mail: [email protected]) Abstract: Volcanism and tectonism are the dominant endogenic means by which planetary sur- faces change. This book, in general, and this overview, in particular, aim to encompass the broad range in character of volcanism, tectonism, faulting and associated interactions observed on plane- tary bodies across the inner solar system – a region that includes Mercury, Venus, Earth, the Moon, Mars and asteroids. The diversity and breadth of landforms produced by volcanic and tectonic pro- cesses are enormous, and vary across the inventory of inner solar system bodies. As a result, the selection of prevailing landforms and their underlying formational processes that are described and highlighted in this review are but a primer to the expansive field of planetary volcanism and tectonism. In addition to this extended introductory contribution, this Special Publication features 21 dedicated research articles about volcanic and tectonic processes manifest across the inner solar system. -

Topographic Map of Mars Any Use of Trade, Product, Or Firm Names in This Publication Is for Descriptive Purposes Only and Does Not Imply Endorsement by the U.S
U.S. DEPARTMENT OF THE INTERIOR Prepared for the GEOLOGIC INVESTIGATIONS SERIES I–2782 U.S. GEOLOGICAL SURVEY NATIONAL AERONAUTICS AND SPACE ADMINISTRATION SHEET 1 OF 2 180° 0° 55° NOTES ON BASE between maps and quadrangles, and most closely resembles lighting conditions –55° This map is based on data from the Mars Orbiter Laser Altimeter (MOLA; found on imagery. The DEM values were then mapped to a smooth global color RussellRussell StokesStokes look-up table. Note that the chosen color scheme simply represents elevation us 150 Smith and others, 2001), an instrument on NASA’s Mars Global Surveyor NOACHISN O A C H I S o E E orousor 30 ° 210 ° E changes and is not intended to imply anything about surface characteristics (for ° p 330 ° E 210 W (MGS) spacecraft (Albee and others, 2001). The image used for the base of this 330 o ° ° W ° W DarwinDarwin lc ° W 150 example, past or current presence of water or ice). These two files were then 30 ha s map represents more than 600 million measurements gathered between 1999 and ChalcopC pe 60° merged and scaled to 1:25 million for the Mercator portion and 1:15,196,708 for –60° u v 2001, adjusted for consistency (Neumann and others, 2001, 2003) and converted R MilankovicMilankovic to planetary radii. These have been converted to elevations above the areoid as the two Polar Stereographic portions, with a resolution of 300 dots per inch. The determined from a martian gravity field solution GMM-2B (Lemoine and others, projections have a common scale of 1:13,923,113 at ±56° latitude. -
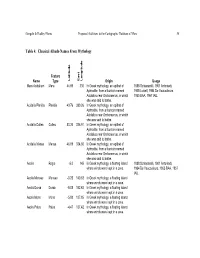
Table 4: Classical Albedo Names from Mythology
Gangale & Dudley-Flores Proposed Additions to the Cartographic Database of Mars 54 Table 4: Classical Albedo Names From Mythology Feature Name Type Latitude East Longitude Origin Usage Mare Acidalium Mare 44.66 330 In Greek mythology, an epithet of 1888 Schiaparelli, 1901 Antoniadi, Aphrodite, from a fountain named 1905 Lowell, 1954 De Vaucouleurs, Acidalius near Orchomenus, in which 1955 BAA, 1967 IAU. she was said to bathe. Acidalia Planitia Planitia 49.76 339.26 In Greek mythology, an epithet of Aphrodite, from a fountain named Acidalius near Orchomenus, in which she was said to bathe. Acidalia Colles Colles 50.34 336.91 In Greek mythology, an epithet of Aphrodite, from a fountain named Acidalius near Orchomenus, in which she was said to bathe. Acidalia Mensa Mensa 46.69 334.66 In Greek mythology, an epithet of Aphrodite, from a fountain named Acidalius near Orchomenus, in which she was said to bathe. Aeolis Regio -5.0 145 In Greek mythology, a floating island 1888 Schiaparelli, 1901 Antoniadi, where winds were kept in a cave. 1954 De Vaucouleurs, 1955 BAA, 1957 IAU. Aeolis Mensae Mensae -3.25 140.63 In Greek mythology, a floating island where winds were kept in a cave. Aeolis Dorsa Dorsa -5.05 152.63 In Greek mythology, a floating island where winds were kept in a cave. Aeolis Mons Mons -5.08 137.85 In Greek mythology, a floating island where winds were kept in a cave. Aeolis Palus Palus -4.47 137.42 In Greek mythology, a floating island where winds were kept in a cave. -

An Overview of Geologic Mapping Results for the Polar Regions of Mars
Fourth Mars Polar Science Conference (2006) 8098.pdf AN OVERVIEW OF GEOLOGIC MAPPING RESULTS FOR THE POLAR REGIONS OF MARS. K.L. Tanaka1 and E.J. Kolb2, 1Astrogeology Team, U.S. Geological Survey, Flagstaff, AZ 86001, [email protected], 2Arizona State University, Tempe, AZ 85287, [email protected]. Introduction: The polar regions of Mars include the Perhaps during much of the Amazonian, dark, densest data coverage for the planet because of the polar (possibly made up of weathered basalt [8]) dune fields orbits of MGS, ODY, and MEX. Because the geology of migrated across the circum-polar plains mainly north of the polar plateaus has been among the most dynamic on 70ºN where dunes are presently common. We map the the planet in recent geologic time, the data enable the current dune fields as the Olympia Undae unit, after the most detailed and complex geologic investigations of any largest dune field. This unit also includes rippled sur- regions on Mars, superseding previous, even recent, map- faces as seen in MOC images that may form an under- ping efforts [e.g., 1-3]. Geologic mapping at regional and lying, indurated sand sheet. Some of the present dune local scales is revealing that the stratigraphy and modifi- fields originate from steep scarps exposed on the mar- cational histories of polar materials by various processes gins of Abalos Mensae, from Boreum and Tenuis Cavi is highly complex at both poles. Here, we describe some at the head of Chasma Boreale, and from reentrants of of our recent results in polar geologic mapping and how Olympia Cavi into Planum Boreum. -

High-Resolution Studies of Aqueous Environments on Ancient Mars
HIGH-RESOLUTION STUDIES OF AQUEOUS ENVIRONMENTS ON ANCIENT MARS A Dissertation Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by James Joseph Wray January 2011 © 2011 James Joseph Wray HIGH-RESOLUTION STUDIES OF AQUEOUS ENVIRONMENTS ON ANCIENT MARS James Joseph Wray, Ph.D. Cornell University 2011 Hydrated minerals on the surface of Mars record past aqueous conditions and permit assessment of whether, where, and when the planet may have been habitable. Both phyllosilicates (e.g., clays) and hydrated sulfate minerals were recently identified via orbital near-infrared spectroscopy. This work uses the Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) and High Resolution Imaging Science Experiment (HiRISE) to characterize these and other aqueous mineral deposits, determining their composition, stratigraphy, and morphology. These properties and observations from other Mars-orbiting instruments allow formulation and testing of hypotheses on how Martian environments varied across space and time. The Mawrth Vallis region hosts the largest areal exposure of phyllosilicates, and CRISM spectral maps show these are compositionally stratified, with Al-clays overlying Fe/Mg-clays throughout the region. Geometric measurements reveal that the Al-clay horizon traces the Mawrth Vallis topography, implying that the Al-clays postdate this channel and may have formed via surface weathering. CRISM data further reveal the Ca-sulfate bassanite in outcrops underlying Fe/Mg-clays. Each hydrated unit exhibits ubiquitous meter-scale polygons or other fracture patterns, which correlate with composition. A CRISM-based survey of Mars’ ancient southern highlands uncovers numerous aqueous deposits undetected at lower resolution.