Ecotypic Variation in Elymus Elymoides Subsp. Brevifolius in the Northern Intermountain West Matthew C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of the Vascular Plants of Redwood National Park
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 9-17-2018 Checklist of the Vascular Plants of Redwood National Park James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Checklist of the Vascular Plants of Redwood National Park" (2018). Botanical Studies. 85. https://digitalcommons.humboldt.edu/botany_jps/85 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A CHECKLIST OF THE VASCULAR PLANTS OF THE REDWOOD NATIONAL & STATE PARKS James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State Univerity Arcata, California 14 September 2018 The Redwood National and State Parks are located in Del Norte and Humboldt counties in coastal northwestern California. The national park was F E R N S established in 1968. In 1994, a cooperative agreement with the California Department of Parks and Recreation added Del Norte Coast, Prairie Creek, Athyriaceae – Lady Fern Family and Jedediah Smith Redwoods state parks to form a single administrative Athyrium filix-femina var. cyclosporum • northwestern lady fern unit. Together they comprise about 133,000 acres (540 km2), including 37 miles of coast line. Almost half of the remaining old growth redwood forests Blechnaceae – Deer Fern Family are protected in these four parks. -

A Comparison of Cumulative-Germination Response of Cheatgrass (Bromus Tectorum L.) and Five Perennial Bunchgrass Species to Simulated Field-Temperature Regimes
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 2010 A comparison of cumulative-germination response of cheatgrass (Bromus tectorum L.) and five perennial bunchgrass species to simulated field-temperature regimes Stuart P. Hardegree USDA-Agricultural Research Service, [email protected] Corey A. Moffet USDA-Agricultural Research Service Bruce A. Roundy Brigham Young University Thomas A. Jones USDA-Agricultural Research Service Stephen J. Novak Boise State University See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Part of the Agricultural Science Commons Hardegree, Stuart P.; Moffet, Corey A.; Roundy, Bruce A.; Jones, Thomas A.; Novak, Stephen J.; Clark, Patrick E.; Pierson, Frederick B.; and Flerchinger, Gerald N., "A comparison of cumulative-germination response of cheatgrass (Bromus tectorum L.) and five perennial bunchgrass species to simulated field- temperature regimes" (2010). Publications from USDA-ARS / UNL Faculty. 855. https://digitalcommons.unl.edu/usdaarsfacpub/855 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University -

Draft Plant Propagation Protocol
Plant Propagation Protocol for Elymus elymoides ESRM 412 – Native Plant Production http://courses.washington.edu/esrm412/protocols/ELEL5.pdf TAXONOMY Family Names Family Scientific Poaceae Name: Family Common True grasses Name: Scientific Names Genus: Elymus Species: Elymoides Species Authority: Swezey Variety: Sub-species: Cultivar: Authority for Variety/Sub- species: Common Sitanion hystrix (Nutt.) J.G. Smith Synonym(s) Elymus hystrix L. var. bigeloviana (Fern.) Bowden (include full Elymus hystrix L. var. histrix scientific names Elymus elymoides (Raf.) Swezey var. brevifolius (J.G. Sm.) Barkworth (e.g., Elymus Elymus elymoides (Raf.) Swezey var. californicus (J.G. Sm.) Barkworth glaucus Buckley), Elymus elymoides (Raf.) Swezey spp. elymoides including variety Elymus elymoides (Raf.) Swezey var. brevifolius (J.G. Sm.) Barkworth or subspecies Elymus elymoides (Raf.) Swezey spp. hordeoides (Suksdorf) Barkworth information) Elymus elymoides (Raf.) Swezey var. brevifolius (J.G. Sm.) Barkworth (7) Common Name(s): Bottlebrush squirreltail; Squirreltail bottlebrush; Squirreltail; Squirrel tail (1) (2) (7) Species Code (as ELEL5 per USDA Plants database): GENERAL INFORMATION Geographical range Ecological Found throughout western North America from Canada to Mexico. Grows distribution in a wide range of habitats, from shadescale communities to alpine tundra to low lands of the Great Basin. (7) Climate and Typically found from 600 to 3,500 meters. It has been documented in elevation range California from 100 m to 4,300 m in elevation. Widespread in the interior regions of western North America at mid to high elevation sites that receive 6 to 14 inches mean annual precipitation. (3) (5) (7) Local habitat and A component of many different community types, including short-grass abundance prairies where it may be associated with Pascopyrum smithii and Aristida purpurea; sagebrush scrub, where it may be associated with Koeleria macrantha; and sagebrush rangelands with Artemisia tridentata. -

Abundances of Coplanted Native Bunchgrasses and Crested Wheatgrass After 13 Years☆,☆☆
Rangeland Ecology & Management 68 (2015) 211–214 Contents lists available at ScienceDirect Rangeland Ecology & Management journal homepage: http://www.elsevier.com/locate/rama Abundances of Coplanted Native Bunchgrasses and Crested Wheatgrass after 13 Years☆,☆☆ Aleta M. Nafus a,⁎, Tony J. Svejcar b,DavidC.Ganskoppc,KirkW.Daviesd a Graduate Student, Oregon State University, Corvallis, OR 97330, USA b Research Leader, U.S. Department of Agriculture, Agricultural Research Service, Burns, OR 97720, USA c Emeritus Rangeland Scientist, U.S. Department of Agriculture, Agricultural Research Service, Burns, OR 97720, USA d Rangeland Scientist, U.S. Department of Agriculture, Agricultural Research Service, Burns, OR 97720, USA article info abstract Keywords: Crested wheatgrass (Agropyron cristatum [L] Gaertm) has been seeded on more than 5 million hectares in Agropyron cristatum western North America because it establishes more readily than native bunchgrasses. Currently, there is restoration substantial interest in reestablishing native species in sagebrush steppe, but efforts to reintroduce native revegetation grasses into crested wheatgrass stands have been largely unsuccessful, and little is known about the sagebrush steppe long-term dynamics of crested wheatgrass/native species mixes. We examined the abundance of crested wheatgrass and seven native sagebrush steppe bunchgrasses planted concurrently at equal low densities in nongrazed and unburned plots. Thirteen years post establishment, crested wheatgrass was the dominant bunchgrass, with a 10-fold increase in density. Idaho fescue (Festuca idahoensis Elmer), Thurber’s needlegrass (Achnatherum thurberianum (Piper) Barkworth), basin wildrye (Leymus cinereus [Scribn. & Merr.] A. Löve), and Sandberg bluegrass (Poa secunda J. Presl) maintained their low planting density, whereas bluebunch wheatgrass (Pseudoroegneria spicata [Pursh] A. -
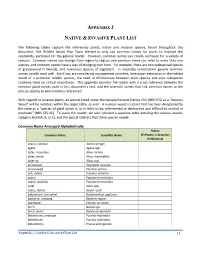
Reference Plant List
APPENDIX J NATIVE & INVASIVE PLANT LIST The following tables capture the referenced plants, native and invasive species, found throughout this document. The Wildlife Action Plan Team elected to only use common names for plants to improve the readability, particular for the general reader. However, common names can create confusion for a variety of reasons. Common names can change from region-to-region; one common name can refer to more than one species; and common names have a way of changing over time. For example, there are two widespread species of greasewood in Nevada, and numerous species of sagebrush. In everyday conversation generic common names usually work well. But if you are considering management activities, landscape restoration or the habitat needs of a particular wildlife species, the need to differentiate between plant species and even subspecies suddenly takes on critical importance. This appendix provides the reader with a cross reference between the common plant names used in this document’s text, and the scientific names that link common names to the precise species to which writers referenced. With regards to invasive plants, all species listed under the Nevada Revised Statute 555 (NRS 555) as a “Noxious Weed” will be notated, within the larger table, as such. A noxious weed is a plant that has been designated by the state as a “species of plant which is, or is likely to be, detrimental or destructive and difficult to control or eradicate” (NRS 555.05). To assist the reader, we also included a separate table detailing the noxious weeds, category level (A, B, or C), and the typical habitats that these species invade. -

Crested Wheatgrass (Agropyron Cristatum) Seedings in Western Colorado: What Can We Learn?
Management of Biological Invasions (2012) Volume 3, Issue 2: 89–96 doi: http://dx.doi.org/10.3391/mbi.2012.3.2.03 Open Access © 2012 The Author(s). Journal compilation © 2012 REABIC Research Article Crested wheatgrass (Agropyron cristatum) seedings in Western Colorado: What can we learn? M. Nikki Grant-Hoffman*, Amanda Clements, Anna Lincoln and James Dollerschell Colorado National Landscape Conservation System, Bureau of Land Management, Grand Junction Field Office, USA E-mail: [email protected] (MNGH), [email protected] (AC), [email protected] (AL), [email protected] (JD) *Corresponding author Received: 17 September 2012 / Accepted: 10 October 2012 / Published online: 15 December 2012 Handling editor: Elias Dana, University of Almeria, Spain Abstract Non-native species have been widely transported, becoming components of ecosystems worldwide. In some cases this can change the structure and function of an ecosystem. Crested wheatgrass (Agropyron cristatum, Agropyron spp.) was introduced into the Western U.S. in the late 18th and early 19th centuries. Since introduction, it has been planted in western rangelands currently occupying millions of acres. Crested wheatgrass causes significant changes in areas where it dominates the vegetation, and restoring rangelands planted with crested wheatgrass to higher plant diversity and ecosystem function has been met with limited success. Here we revisit historical frequency monitoring data collected in western Colorado on public lands that were planted with crested wheatgrass between 1940 and 1980. We also monitored vegetation before and after mechanical treatment (removal of vegetation with the use of a dixie harrow pulled behind a tractor) and re-seeding of desirable species in three areas dominated by crested wheatgrass. -

Appendix 6 Biological Report (PDF)
Biological Constraints Analysis Tahoe Donner 5-Year Trail Implementation Plan Truckee, Nevada County, CA Nevada County File Number ___ Prepared for: Tahoe Donner Association Forrest Huisman, Director of Capital Projects 11509 Northwoods Boulevard Truckee, California 96161 530-587-9487 Prepared by: Micki Kelly Kelly Biological Consulting PO Box 1625 Truckee, CA 96160 530-582-9713 June 2015, Revised December 2015 Biological Constraints Report, Tahoe Donner Trails 5-Year Implementation Plan December 2015 Table of Contents 1.0 INFORMATION SUMMARY ..................................................................................................................................... 1 2.0 PROJECT AND PROPERTY DESCRIPTION ................................................................................................................. 4 2.1 SITE OVERVIEW ............................................................................................................................................................ 4 2.2 REGULATORY FRAMEWORK ............................................................................................................................................. 4 2.2.1 Special-Status Species ...................................................................................................................................... 5 2.2.2 Wetlands and Waters of the U.S. ..................................................................................................................... 6 2.2.3 Waters of the State ......................................................................................................................................... -

Medusahead Management Guide for the Western States. University of California, Weed Research and Information Center, Davis
#822 Medusahead Management Guide for the Western States GUY B. KYSER Weed Science Program Department of Plant Sciences University of California, Davis, CA JOSEPH M. DITOMASO Weed Science Program Department of Plant Sciences University of California, Davis, CA KIRK W. DAVIES Eastern Oregon Agricultural Research Center, Burns, OR JOSH S. DAVY University of California Cooperative Extension, Tehama County, CA BRENDA S. SMITH Eastern Oregon Agricultural Research Center, Burns, OR Medusahead Management Guide for the Western States Guy B. Kyser Weed Science Program, Department of Plant Sciences University of California, Davis, CA Joseph M. DiTomaso Weed Science Program, Department of Plant Sciences University of California, Davis, CA Kirk W. Davies Eastern Oregon Agricultural Research Center, Burns, OR Josh S. Davy University of California Cooperative Extension, Tehama County, CA Brenda S. Smith Eastern Oregon Agricultural Research Center, Burns, OR Published by the Weed Research and Information Center, University of California. Recommended citation: Kyser GB, DiTomaso JM, Davies KW, Davy JS, Smith BS (2014) Medusahead Management Guide for the Western States. University of California, Weed Research and Information Center, Davis. 68 p. Available at: wric.ucdavis.edu. Acknowledgements WE ARE INDEBTED TO James A. Young, USDA- Neil McDougald,IUniversity of California Coopera- ARS, Reno, NV (retired), for his wide-ranging body tive Extension Farm Advisor, Madera County of work with medusahead, and for his enjoyable, lit- Melissa Merrill-Davies, University of California Co- erate science writing. In particular, his 1992 review of operative Extension County Director and Farm medusahead ecology and management was indispen- Advisor, Modoc County sable in preparation of this manuscript. -

Resource Selection, and Demographic Rates of Female Greater Sage-Grouse Following Large-Scale Wildfire
AN ABSTRACT OF THE THESIS OF Lee Foster for the degree of Master of Science in Wildlife Science presented on May 31, 2016 Title: Resource Selection and Demographic Rates of Female Greater Sage-Grouse Following Large-Scale Wildfire Abstract Approved: Katie. M. Dugger, Christian A. Hagen Understanding the effects of habitat disturbance on a species’ habitat selection patterns, and demographic rates, is essential to projecting the trajectories of populations affected by disturbance, as well as for determining the appropriate conservation actions needed to maintain those populations. Greater sage-grouse (Centrocercus urophasianus) is a species of conservation concern in western North America. The distribution of the species has been reduced by approximately half since European settlement, with concurrent and continuing population declines across its occupied range. The primary threats to the species are habitat alteration and loss, caused by multiple factors. In the western portion of its distribution, increasing wildfire activity is a primary cause of habitat loss and degradation. Single wildfires in this area may now reach extremely large sizes (>100,000 ha), and wildfires have been linked to local population declines. However, no published studies, to date, have examined the immediate effects of large-scale wildfire on sage-grouse habitat selection and demographic rates, using modern telemetry methods. I studied the habitat selection patterns, nest success, and survival of adult, and yearling female sage-grouse, captured within or near the Holloway fire, using state- of-the-art GPS-PTT telemetry methods. The Holloway fire burned ~187,000 ha of highly productive sage-grouse habitat in August, 2012. My study began during the first spring post-fire (March, 2013), and continued through February, 2015. -
![EVALUATION and ENHANCEMENT of SEED LOT QUALITY in EASTERN GAMAGRASS [Tripsacum Dactyloides (L.) L.]](https://docslib.b-cdn.net/cover/1098/evaluation-and-enhancement-of-seed-lot-quality-in-eastern-gamagrass-tripsacum-dactyloides-l-l-2631098.webp)
EVALUATION and ENHANCEMENT of SEED LOT QUALITY in EASTERN GAMAGRASS [Tripsacum Dactyloides (L.) L.]
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2010 EVALUATION AND ENHANCEMENT OF SEED LOT QUALITY IN EASTERN GAMAGRASS [Tripsacum dactyloides (L.) L.] Cynthia Hensley Finneseth University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Finneseth, Cynthia Hensley, "EVALUATION AND ENHANCEMENT OF SEED LOT QUALITY IN EASTERN GAMAGRASS [Tripsacum dactyloides (L.) L.]" (2010). University of Kentucky Doctoral Dissertations. 112. https://uknowledge.uky.edu/gradschool_diss/112 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Cynthia Hensley Finneseth The Graduate School University of Kentucky 2010 EVALUATION AND ENHANCEMENT OF SEED LOT QUALITY IN EASTERN GAMAGRASS [Tripsacum dactyloides (L.) L.] _________________________________ ABSTRACT OF DISSERTATION _________________________________ A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Agriculture at the University of Kentucky By Cynthia Hensley Finneseth Lexington, Kentucky Director: Dr. Robert Geneve, Professor of Horticulture Lexington, Kentucky 2010 Copyright © Cynthia Hensley Finneseth 2010 ABSTRACT OF DISSERTATION EVALUATION AND ENHANCEMENT OF SEED LOT QUALITY IN EASTERN GAMAGRASS [Tripsacum dactyloides (L.) L.] Eastern gamagrass [Tripsacum dactyloides (L.) L.] is a warm-season, perennial grass which is native to large areas across North America. Cultivars, selections and ecotypes suitable for erosion control, wildlife planting, ornamental, forage and biofuel applications are commercially available. -

Established Native Perennial Grasses Out-Compete an Invasive Annual Grass Regardless of Soil Water and Nutrient Availability
Plant Ecol DOI 10.1007/s11258-011-9992-1 Established native perennial grasses out-compete an invasive annual grass regardless of soil water and nutrient availability Christopher M. McGlone • Carolyn Hull Sieg • Thomas E. Kolb • Ty Nietupsky Received: 21 December 2010 / Accepted: 6 October 2011 Ó Springer Science+Business Media B.V. 2011 Abstract Competition and resource availability influ- grasses were highly competitive against B. tectorum, ence invasions into native perennial grasslands by non- regardless of water, N, or P availability. Bromus native annual grasses such as Bromus tectorum.Intwo tectorum reproductive potential (viable seed produc- greenhouse experiments we examined the influence of tion) was not significantly influenced by any experi- competition, water availability, and elevated nitrogen mental manipulation, except for competition with P. (N) and phosphorus (P) availability on growth and smithii. In all cases, B. tectorum per-plant production of reproduction of the invasive annual grass B. tectorum viable seeds exceeded parental replacement. Our results and two native perennial grasses (Elymus elymoides, show that established plants of Elymus elymoides Pascopyrum smithii). Bromus tectorum aboveground and Pascopyrum smithii compete successfully against biomass and seed production were significantly reduced B. tectorum over a wide range of soil resource when grown with one or more established native availability. perennial grasses. Conversely, average seed weight and germination were significantly lower in the B. Keywords Bromus tectorum Á Competition Á tectorum monoculture than in competition native Greenhouse Á Nitrogen Á Phosphorus Á perennial grasses. Intraspecific competition reduced Water availability per-plant production of both established native grasses, whereas interspecific competition from B. tectorum increased production. -

Intraspecific Variation, Adaptation, and Evolution Chapter Author(S): Ryan E
University of California Press Chapter Title: Intraspecific Variation, Adaptation, and Evolution Chapter Author(s): Ryan E. O’Dell and Nishanta Rajakaruna Book Title: Serpentine Book Subtitle: The Evolution and Ecology of a Model System Book Editor(s): Susan Harrison, Nishanta Rajakaruna Published by: University of California Press. (2011) Stable URL: https://www.jstor.org/stable/10.1525/j.ctt1pnqkb.10 JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at https://about.jstor.org/terms University of California Press is collaborating with JSTOR to digitize, preserve and extend access to Serpentine This content downloaded from 129.65.41.106 on Wed, 16 Oct 2019 01:33:07 UTC All use subject to https://about.jstor.org/terms 5 Intraspecific Variation, Adaptation, and Evolution Ryan E. O’Dell, Bureau of Land Management Nishanta Rajakaruna, College of the Atlantic Intraspecific variation refers to the genotypic (genetic) and resulting phenotypic (morphological and physiological) variation found within a species. Variation within a species is crucial for adaptation and evolution by natural selection. Over time, selection can result in genetically distinct populations of a species that are adapted to specific environmental conditions. Such populations are referred to as ecotypes. The study of distinct climatic, elevational, latitudinal, geographic, and edaphic ecotypes has provided much insight into the process of evolution by natu- ral selection (Briggs and Walters, 1997; Levin, 2000; Silvertown and Charlesworth, 2001).