A Comparison of Cumulative-Germination Response of Cheatgrass (Bromus Tectorum L.) and Five Perennial Bunchgrass Species to Simulated Field-Temperature Regimes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apparent Competition with Bromus Tectorum Through Pyrenophora Semeniperda Reduces Establishment of Native Grasses
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2011-03-16 Apparent Competition with Bromus tectorum Through Pyrenophora semeniperda Reduces Establishment of Native Grasses Katherine Temus Merrill Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Animal Sciences Commons BYU ScholarsArchive Citation Merrill, Katherine Temus, "Apparent Competition with Bromus tectorum Through Pyrenophora semeniperda Reduces Establishment of Native Grasses" (2011). Theses and Dissertations. 2956. https://scholarsarchive.byu.edu/etd/2956 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Apparent competition with Bromus tectorum through Pyrenophora semeniperda reduces establishment of native grasses Katherine T. Merrill A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Phil S. Allen, Chair Susan E. Meyer Samuel B. St. Clair Department of Plant and Wildlife Sciences Brigham Young University April 2011 Copyright © 2010 Katherine T. Merrill All Rights Reserved ABSTRACT Apparent competition with Bromus tectorum through Pyrenophora semeniperda reduces establishment of native grasses Katherine T. Merrill Department of Plant and Wildlife Sciences, BYU Master of Science Contributing to the success of Bromus tectorum in the Intermountain West may be a mechanism called apparent competition, which occurs when one species increases the pressure of a consumer on a second species. This indirect interaction has been documented only a few times in invasive plant systems, and never in a fungal pathosystem. -

Linking Physiological Traits and Species Abundance to Invasion Resistance
Linking physiological traits and species abundance to invasion resistance Jeremy James Framework • Plant community composition influences ecosystem properties • Much emphasis on effects of functional group diversity on ecosystem properties • Invasive plant management can be improved by managing plant communities based on functional traits as opposed to functional groups Outline • An example of how functional traits influence ecosystem properties (N capture and invasion) – Traits related to N capture – Trait effects on ecosystems are moderated by species abundance • What traits might be important to consider when revegetating areas prone to weed invasion? – At the seedling stage traits affecting initial growth rate important • Conclusions and future directions Functional group diversity, nitrogen capture and invasion resistance Study site Group Code Common Name Scientific Name Annual BRTE cheatgrass Bromus tectorum L. Annual TACA medusahead Taeniatherum caput-medusae (L.) Nevski Bunchgrass PSSP bluebunch wheatgrass Pseudoroegneria spicata (Pursh) A. Löve Bunchgrass ELEL bottlebrush squirreltail Elymus elymoides (Raf.) Swezey Bunchgrass POSE Sandberg’s bluegrass Poa secunda J. Presl Forb LOTR nineleaf biscuitroot Lomatium triternatum (Pursh) Coult. & Rose Forb CRIN grey hawksbeard Crepis intermedia Gray Experimental design • 15N was injected into soils around 7 study species • Injections were made: – 3 times during the growing season (April, May June) – At 2 soil depth (2-7 cm, 17-22 cm) + - – Using 2 forms of N (NH4 , NO3 ) • Removal plots -

Predicting Foundation Bunchgrass Species Abundances: Model-Assisted Decision-Making in Protected-Area Sagebrush Steppe 1, 2 3 3 4 THOMAS J
#825 Predicting foundation bunchgrass species abundances: model-assisted decision-making in protected-area sagebrush steppe 1, 2 3 3 4 THOMAS J. RODHOUSE, KATHRYN M. IRVINE, ROGER L. SHELEY, BRENDA S. SMITH, SHIRLEY HOH, 5 5 DANIEL M. ESPOSITO, AND RICARDO MATA-GONZALEZ 1Upper Columbia Basin Network, National Park Service, 63095 Deschutes Market Road, Bend, Oregon 97701 USA 2Northern Rocky Mountain Science Center, United States Geological Survey, 2327 University Way, Suite 2, Bozeman, Montana 59715 USA 3Agricultural Research Service, United States Department of Agriculture, 67826-A, Highway 205, Burns, Oregon 97720 USA 4John Day Fossil Beds National Monument, National Park Service, 32651 Highway 19, Kimberly, Oregon 97848 USA 5Department of Animal and Rangeland Sciences, Oregon State University, Corvallis, Oregon 97331 USA Citation: Rodhouse, T. J., K. M. Irvine, R. L. Sheley, B. S. Smith, S. Hoh, D. M. Esposito, and R. Mata-Gonzalez. 2014. Predicting foundation bunchgrass species abundances: model-assisted decision-making in protected-area sagebrush steppe. Ecosphere 5(9):108. http://dx.doi.org/10.1890/ES14-00169.1 Abstract. Foundation species are structurally dominant members of ecological communities that can stabilize ecological processes and influence resilience to disturbance and resistance to invasion. Being common, they are often overlooked for conservation but are increasingly threatened from land use change, biological invasions, and over-exploitation. The pattern of foundation species abundances over space and time may be used to guide decision-making, particularly in protected areas for which they are iconic. We used ordinal logistic regression to identify the important environmental influences on the abundance patterns of bluebunch wheatgrass (Pseudoroegneria spicata), Thurber’s needlegrass (Achnatherum thurber- ianum), and Sandberg bluegrass (Poa secunda) in protected-area sagebrush steppe. -

INDIAN RICEGRASS Erosion Control/Reclamation: One of Indian Ricegrass' Greatest Values Is for Stabilizing Sites Susceptible to Achnatherum Hymenoides Wind Erosion
Plant Guide INDIAN RICEGRASS Erosion control/reclamation: One of Indian ricegrass' greatest values is for stabilizing sites susceptible to Achnatherum hymenoides wind erosion. It is well adapted to stabilization of (Roemer & J.A. Schultes) disturbed sandy soils in mixes with other species. It is naturally an early invader onto disturbed sandy Barkworth sites (after and in concert with needle and thread Plant Symbol = ACHY grass). It is also one of the first to establish on cut and fill slopes. It does not compete well with Contributed By: USDA NRCS Idaho State Office aggressive introduced grasses during the establishment period, but is very compatible with slower developing natives, such as Snake River wheatgrass (Elymus wawawaiensis), bluebunch wheatgrass (Pseudoroegneria spicata), thickspike wheatgrass (Elymus lanceolata ssp. lanceolata), streambank wheatgrass (Elymus lanceolata ssp. psammophila), western wheatgrass (Pascopyrum smithii), and needlegrass species (Stipa spp. and Ptilagrostis spp.). Drought tolerance combined with fibrous root system and fair to good seedling vigor, make Indian ricegrass desirable for reclamation in areas receiving 8 to 14 inches annual precipitation. Wildlife: Forage value is mentioned in the grazing/rangeland/hayland section above. Due to the abundance of plump, nutritious seed produced by Indian ricegrass, it is considered an excellent food source for birds, such as morning doves, pheasants, and songbirds. Rodents collect the seed for winter food supplies. It is considered good cover habitat for small -

Draft Plant Propagation Protocol
Plant Propagation Protocol for Elymus elymoides ESRM 412 – Native Plant Production http://courses.washington.edu/esrm412/protocols/ELEL5.pdf TAXONOMY Family Names Family Scientific Poaceae Name: Family Common True grasses Name: Scientific Names Genus: Elymus Species: Elymoides Species Authority: Swezey Variety: Sub-species: Cultivar: Authority for Variety/Sub- species: Common Sitanion hystrix (Nutt.) J.G. Smith Synonym(s) Elymus hystrix L. var. bigeloviana (Fern.) Bowden (include full Elymus hystrix L. var. histrix scientific names Elymus elymoides (Raf.) Swezey var. brevifolius (J.G. Sm.) Barkworth (e.g., Elymus Elymus elymoides (Raf.) Swezey var. californicus (J.G. Sm.) Barkworth glaucus Buckley), Elymus elymoides (Raf.) Swezey spp. elymoides including variety Elymus elymoides (Raf.) Swezey var. brevifolius (J.G. Sm.) Barkworth or subspecies Elymus elymoides (Raf.) Swezey spp. hordeoides (Suksdorf) Barkworth information) Elymus elymoides (Raf.) Swezey var. brevifolius (J.G. Sm.) Barkworth (7) Common Name(s): Bottlebrush squirreltail; Squirreltail bottlebrush; Squirreltail; Squirrel tail (1) (2) (7) Species Code (as ELEL5 per USDA Plants database): GENERAL INFORMATION Geographical range Ecological Found throughout western North America from Canada to Mexico. Grows distribution in a wide range of habitats, from shadescale communities to alpine tundra to low lands of the Great Basin. (7) Climate and Typically found from 600 to 3,500 meters. It has been documented in elevation range California from 100 m to 4,300 m in elevation. Widespread in the interior regions of western North America at mid to high elevation sites that receive 6 to 14 inches mean annual precipitation. (3) (5) (7) Local habitat and A component of many different community types, including short-grass abundance prairies where it may be associated with Pascopyrum smithii and Aristida purpurea; sagebrush scrub, where it may be associated with Koeleria macrantha; and sagebrush rangelands with Artemisia tridentata. -

Abundances of Coplanted Native Bunchgrasses and Crested Wheatgrass After 13 Years, Author(S): Aleta M
#843 Abundances of Coplanted Native Bunchgrasses and Crested Wheatgrass after 13 Years, Author(s): Aleta M. Nafus, Tony J. Svejcar, David C. Ganskopp and Kirk W. Davies Source: Rangeland Ecology & Management, 68(2):211-214. Published By: Society for Range Management URL: http://www.bioone.org/doi/full/10.1016/j.rama.2015.01.011 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. #843 Rangeland Ecology & Management 68 (2015) 211–214 Contents lists available at ScienceDirect Rangeland Ecology & Management journal homepage: http://www.elsevier.com/locate/rama Abundances of Coplanted Native Bunchgrasses and Crested Wheatgrass after 13 Years☆,☆☆ Aleta M. Nafus a,⁎, Tony J. Svejcar b,DavidC.Ganskoppc,KirkW.Daviesd a Graduate Student, Oregon State University, Corvallis, OR 97330, USA b Research Leader, U.S. Department of Agriculture, Agricultural Research Service, Burns, OR 97720, USA c Emeritus Rangeland Scientist, U.S. -

Abundances of Coplanted Native Bunchgrasses and Crested Wheatgrass After 13 Years☆,☆☆
Rangeland Ecology & Management 68 (2015) 211–214 Contents lists available at ScienceDirect Rangeland Ecology & Management journal homepage: http://www.elsevier.com/locate/rama Abundances of Coplanted Native Bunchgrasses and Crested Wheatgrass after 13 Years☆,☆☆ Aleta M. Nafus a,⁎, Tony J. Svejcar b,DavidC.Ganskoppc,KirkW.Daviesd a Graduate Student, Oregon State University, Corvallis, OR 97330, USA b Research Leader, U.S. Department of Agriculture, Agricultural Research Service, Burns, OR 97720, USA c Emeritus Rangeland Scientist, U.S. Department of Agriculture, Agricultural Research Service, Burns, OR 97720, USA d Rangeland Scientist, U.S. Department of Agriculture, Agricultural Research Service, Burns, OR 97720, USA article info abstract Keywords: Crested wheatgrass (Agropyron cristatum [L] Gaertm) has been seeded on more than 5 million hectares in Agropyron cristatum western North America because it establishes more readily than native bunchgrasses. Currently, there is restoration substantial interest in reestablishing native species in sagebrush steppe, but efforts to reintroduce native revegetation grasses into crested wheatgrass stands have been largely unsuccessful, and little is known about the sagebrush steppe long-term dynamics of crested wheatgrass/native species mixes. We examined the abundance of crested wheatgrass and seven native sagebrush steppe bunchgrasses planted concurrently at equal low densities in nongrazed and unburned plots. Thirteen years post establishment, crested wheatgrass was the dominant bunchgrass, with a 10-fold increase in density. Idaho fescue (Festuca idahoensis Elmer), Thurber’s needlegrass (Achnatherum thurberianum (Piper) Barkworth), basin wildrye (Leymus cinereus [Scribn. & Merr.] A. Löve), and Sandberg bluegrass (Poa secunda J. Presl) maintained their low planting density, whereas bluebunch wheatgrass (Pseudoroegneria spicata [Pursh] A. -

Literature Cited Robert W. Kiger, Editor This Is a Consolidated List Of
RWKiger 26 Jul 18 Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volumes 24 and 25. In citations of articles, the titles of serials are rendered in the forms recommended in G. D. R. Bridson and E. R. Smith (1991). When those forms are abbreviated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix "a"; thus, the sequence of explicit suffixes begins with "b". Works missing from any suffixed sequence here are ones cited elsewhere in the Flora that are not pertinent in these volumes. Aares, E., M. Nurminiemi, and C. Brochmann. 2000. Incongruent phylogeographies in spite of similar morphology, ecology, and distribution: Phippsia algida and P. concinna (Poaceae) in the North Atlantic region. Pl. Syst. Evol. 220: 241–261. Abh. Senckenberg. Naturf. Ges. = Abhandlungen herausgegeben von der Senckenbergischen naturforschenden Gesellschaft. Acta Biol. Cracov., Ser. Bot. = Acta Biologica Cracoviensia. Series Botanica. Acta Horti Bot. Prag. = Acta Horti Botanici Pragensis. Acta Phytotax. Geobot. = Acta Phytotaxonomica et Geobotanica. [Shokubutsu Bunrui Chiri.] Acta Phytotax. -
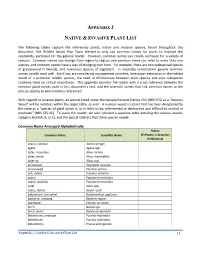
Reference Plant List
APPENDIX J NATIVE & INVASIVE PLANT LIST The following tables capture the referenced plants, native and invasive species, found throughout this document. The Wildlife Action Plan Team elected to only use common names for plants to improve the readability, particular for the general reader. However, common names can create confusion for a variety of reasons. Common names can change from region-to-region; one common name can refer to more than one species; and common names have a way of changing over time. For example, there are two widespread species of greasewood in Nevada, and numerous species of sagebrush. In everyday conversation generic common names usually work well. But if you are considering management activities, landscape restoration or the habitat needs of a particular wildlife species, the need to differentiate between plant species and even subspecies suddenly takes on critical importance. This appendix provides the reader with a cross reference between the common plant names used in this document’s text, and the scientific names that link common names to the precise species to which writers referenced. With regards to invasive plants, all species listed under the Nevada Revised Statute 555 (NRS 555) as a “Noxious Weed” will be notated, within the larger table, as such. A noxious weed is a plant that has been designated by the state as a “species of plant which is, or is likely to be, detrimental or destructive and difficult to control or eradicate” (NRS 555.05). To assist the reader, we also included a separate table detailing the noxious weeds, category level (A, B, or C), and the typical habitats that these species invade. -

A Study of Morphological Variation Within Pseudoroegneria Spicata
AN ABSTRACT OF THE THESIS OF Jack Revnold Carison for the degree of Master of Science in Crop Science presented on March 2O 1986 TITLE: . !st St f p soicata (Pursh) A. L&ve (Poaceae: TriticeaeJ Abstract Approved: Signature redacted for privacy. Robert J. Metzger Chromosome counts were determined for 152 accessions of Pseudo- roegneria spicata (Pursh) A. L6ve and, combined with existing count data, used to plot the distribution of diploid and tetraploid popula- tions. Morphological variation of 55 characters was examined in five groups totaling 205 operational taxonomic units (OTU's), using cluster, principal factor, and discriminant analyses. The five groups included diploid and autotetraploid spicata, an allotetraploid pre- viously included in spicata, a control group including four Old World Pseudoroegneria species, and a small control sample of Elymus lanceolatus (Scrib. and Smith) Gould. The analyses were not able to separate diploid from autoploid spicata nor identify any clear sub- groupings within the dip].ojds. However, the alloploid was separated from spicata and aligned with Elymus lanceolatus based on glume and spike characters. This study recommends the alloploid be included in lanceolatus as a new subspecies, Elymus lanceolatus ssp. wawawai. The chromosome count data indicate it is distributed in the canyons and tributaries of the lower Salmon and Snake Rivers of northern Idaho, northeastern Oregon, and southeastern Washington. The new subspecies keys to Elymus lanceolatus based on glume characters and is separated from subspecies .ianceolatus and albicans by its cespitose growth habit. A S11LPY OF MORPHOLOGICAL VARIATION WITHIN PSELVOROEGNERIA SPICATA (PURSH) A. LOVE (POACEAE: TRITWEAE) By Jack Reynold Carison A THESIS Submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed March 20, 1986 Commencement June, 1986 APPROVED: 1' Signature redacted for privacy. -

Crested Wheatgrass (Agropyron Cristatum) Seedings in Western Colorado: What Can We Learn?
Management of Biological Invasions (2012) Volume 3, Issue 2: 89–96 doi: http://dx.doi.org/10.3391/mbi.2012.3.2.03 Open Access © 2012 The Author(s). Journal compilation © 2012 REABIC Research Article Crested wheatgrass (Agropyron cristatum) seedings in Western Colorado: What can we learn? M. Nikki Grant-Hoffman*, Amanda Clements, Anna Lincoln and James Dollerschell Colorado National Landscape Conservation System, Bureau of Land Management, Grand Junction Field Office, USA E-mail: [email protected] (MNGH), [email protected] (AC), [email protected] (AL), [email protected] (JD) *Corresponding author Received: 17 September 2012 / Accepted: 10 October 2012 / Published online: 15 December 2012 Handling editor: Elias Dana, University of Almeria, Spain Abstract Non-native species have been widely transported, becoming components of ecosystems worldwide. In some cases this can change the structure and function of an ecosystem. Crested wheatgrass (Agropyron cristatum, Agropyron spp.) was introduced into the Western U.S. in the late 18th and early 19th centuries. Since introduction, it has been planted in western rangelands currently occupying millions of acres. Crested wheatgrass causes significant changes in areas where it dominates the vegetation, and restoring rangelands planted with crested wheatgrass to higher plant diversity and ecosystem function has been met with limited success. Here we revisit historical frequency monitoring data collected in western Colorado on public lands that were planted with crested wheatgrass between 1940 and 1980. We also monitored vegetation before and after mechanical treatment (removal of vegetation with the use of a dixie harrow pulled behind a tractor) and re-seeding of desirable species in three areas dominated by crested wheatgrass. -

Chapter 2. Ecosystem Profile
CHAPTER 2. ECOSYSTEM PROFILE 2.1 Introduction Kittitas County is situated in central Washington on the eastern slopes of the Cascade Mountains, between the Cascade Crest and the Columbia River in the Columbia River basin. The county is contained within three major basins: Upper Yakima (Water Resource Inventory Area [WRIA] 39), Alkali – Squilchuck (WRIA 40), and Naches basin (WRIA 38). Of the 2,297 square miles that constitutes Kittitas County, the majority, 78 percent, lies within the Upper Yakima basin (WRIA 39), which drains into the Yakima River. The Alkali – Squilchuck basin (WRIA 40) comprises 17 percent of the county in the eastern portion and drains into the Columbia River. The remaining 5 percent of the county is contained in the Naches basin (WRIA 38) on its southwestern edge and drains into the Little Naches River, which becomes the Naches River joining the Yakima River in Yakima County. Figure 2-1 shows the locations of the WRIAs in Kittitas County. Four different ecoregions are found within Kittitas County: North Cascades, Cascades, Eastern Cascades Slopes and Foothills, and Columbia Plateau (Figure 2-2). The North Cascades ecoregion, found in the northwestern portion of the county, is characterized by glaciated valleys and narrow-crested ridges punctuated by rugged, high relief peaks approaching 8,000 feet above mean sea level (AMSL). It is forested with fir, hemlock, and, in the drier eastern margins, pine. The Cascades ecoregion, located in southwestern Kittitas County, is similar to the North Cascades, but in contrast has more gently undulating terrain, the climate is more temperate, and there is less occurrence of ponderosa pine.