University of Kwazulu-Natal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B-E.00353.Pdf
© University of Hamburg 2018 All rights reserved Klaus Hess Publishers Göttingen & Windhoek www.k-hess-verlag.de ISBN: 978-3-933117-95-3 (Germany), 978-99916-57-43-1 (Namibia) Language editing: Will Simonson (Cambridge), and Proofreading Pal Translation of abstracts to Portuguese: Ana Filipa Guerra Silva Gomes da Piedade Page desing & layout: Marit Arnold, Klaus A. Hess, Ria Henning-Lohmann Cover photographs: front: Thunderstorm approaching a village on the Angolan Central Plateau (Rasmus Revermann) back: Fire in the miombo woodlands, Zambia (David Parduhn) Cover Design: Ria Henning-Lohmann ISSN 1613-9801 Printed in Germany Suggestion for citations: Volume: Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J. & Jürgens, N. (eds.) (2018) Climate change and adaptive land management in southern Africa – assessments, changes, challenges, and solutions. Biodiversity & Ecology, 6, Klaus Hess Publishers, Göttingen & Windhoek. Articles (example): Archer, E., Engelbrecht, F., Hänsler, A., Landman, W., Tadross, M. & Helmschrot, J. (2018) Seasonal prediction and regional climate projections for southern Africa. In: Climate change and adaptive land management in southern Africa – assessments, changes, challenges, and solutions (ed. by Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J. & Jürgens, N.), pp. 14–21, Biodiversity & Ecology, 6, Klaus Hess Publishers, Göttingen & Windhoek. Corrections brought to our attention will be published at the following location: http://www.biodiversity-plants.de/biodivers_ecol/biodivers_ecol.php Biodiversity & Ecology Journal of the Division Biodiversity, Evolution and Ecology of Plants, Institute for Plant Science and Microbiology, University of Hamburg Volume 6: Climate change and adaptive land management in southern Africa Assessments, changes, challenges, and solutions Edited by Rasmus Revermann1, Kristin M. -

Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi
YIKA-VWAZA TRUST RESEARCH STUDY REPORT N (2017/18) Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi By Sopani Sichinga ([email protected]) September , 2019 ABSTRACT In 2018 – 19, a survey on vascular plants was conducted in Vwaza Marsh Wildlife Reserve. The reserve is located in the north-western Malawi, covering an area of about 986 km2. Based on this survey, a total of 461 species from 76 families were recorded (i.e. 454 Angiosperms and 7 Pteridophyta). Of the total species recorded, 19 are exotics (of which 4 are reported to be invasive) while 1 species is considered threatened. The most dominant families were Fabaceae (80 species representing 17. 4%), Poaceae (53 species representing 11.5%), Rubiaceae (27 species representing 5.9 %), and Euphorbiaceae (24 species representing 5.2%). The annotated checklist includes scientific names, habit, habitat types and IUCN Red List status and is presented in section 5. i ACKNOLEDGEMENTS First and foremost, let me thank the Nyika–Vwaza Trust (UK) for funding this work. Without their financial support, this work would have not been materialized. The Department of National Parks and Wildlife (DNPW) Malawi through its Regional Office (N) is also thanked for the logistical support and accommodation throughout the entire study. Special thanks are due to my supervisor - Mr. George Zwide Nxumayo for his invaluable guidance. Mr. Thom McShane should also be thanked in a special way for sharing me some information, and sending me some documents about Vwaza which have contributed a lot to the success of this work. I extend my sincere thanks to the Vwaza Research Unit team for their assistance, especially during the field work. -

Use of Native Tree Species by an Hispanic Community in Panama Author(S): Salomón Aguilar and Richard Condit Source: Economic Botany, Vol
Use of Native Tree Species by an Hispanic Community in Panama Author(s): Salomón Aguilar and Richard Condit Source: Economic Botany, Vol. 55, No. 2 (Apr. - Jun., 2001), pp. 223-235 Published by: Springer on behalf of New York Botanical Garden Press Stable URL: http://www.jstor.org/stable/4256423 . Accessed: 29/07/2011 18:56 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at . http://www.jstor.org/action/showPublisher?publisherCode=nybg. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. New York Botanical Garden Press and Springer are collaborating with JSTOR to digitize, preserve and extend access to Economic Botany. http://www.jstor.org USE OF NATIVE TREE SPECIES BY AN HISPANIC COMMUNITY IN PANAMA1 SALOMON AGUILAR AND RICHARD CONDIT Aguilar, Salomon and Richard Condit (Centerfor Tropical Forest Science, Smithsonian TropicalResearch Institute,Unit 0948, APO AA 34002-0948 USA;fax 507-212-8148 (Pana- ma); email [email protected]). -

Chemical Constituents and Biological Activities of Zanthoxylum Limonella (Rutaceae): a Review
Supabphol & Tangjitjareonkun Tropical Journal of Pharmaceutical Research December 2014; 13 (12): 2119-2130 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v13i12.25 Review Article Chemical Constituents and Biological Activities of Zanthoxylum limonella (Rutaceae): A Review Roongtawan Supabphol1 and Janpen Tangjitjareonkun2* 1Department of Physiology, Faculty of Medicine, Srinakharinwirot University, Bangkok 10110, 2Department of Basic Science and Physical Education, Faculty of Science at Si Racha, Kasetsart University, Si Racha Campus, Chonburi 20230, Thailand *For correspondence: Email: [email protected] Received: 9 December 2013 Revised accepted: 20 October 2014 Abstract Zanthoxylum limonella belongs to the family of aromatic deciduous trees and shrubs, Rutaceae. In traditional medicine practice, various parts of Z. limonella are used for the treatment of dental caries, febrifugal, sudorific, rheumatism, diuretic, stomach ache and diarrhea. Secondary metabolites have been isolated the stems, stem barks, and fruits. The plant contains alkaloid, amide, lignin, coumarin and terpenoid compounds. The extracts of the various parts, essential oil from the fruits and some pure compounds of Z. limonella have been found to have biological activities, for example, mosquito repellent and mosquito larvicidal, antimicrobial, antioxidant, and antitumour properties. -

Boigu Islands, Form the Northern Island Group of Torres Strait, Located Approximately 150 Km North of Thursday Island (See Figure 1)
PROFILE FOR MANAGEMENT OF THE HABITATS AND RELATED ECOLOGICAL AND CULTURAL RESOURCE VALUES OF DAUAN ISLAND January 2013 Prepared by 3D Environmental for Torres Strait Regional Authority Land & Sea Management Unit Cover image: 3D Environmental (2013) EXECUTIVE SUMMARY The granite rock pile that forms Dauan, along with nearby Saibai and Boigu Islands, form the Northern Island Group of Torres Strait, located approximately 150 km north of Thursday Island (see Figure 1). Whilst Saibai and Boigu Island are extensions of the alluvial Fly Platform, geologically part of the Papua New Guinea mainland, Dauan is formed on continental basement rock which extends northward from Cape York Peninsula to Mabadauan Hill on the south-west coast of Papua New Guinea. A total of 14 vegetation communities, within ten broad vegetation groups and 14 regional ecosystems are recognised on the island. The total known flora of comprises 402 species (14 ferns, 388 angiosperms), with 317 native and 85 naturalised species. Nine plant species are considered threatened at the commonwealth and state levels and a further 25 species considered to have significance at a regional level. As for the majority of Torres Strait Islands there is a lack of systematic survey of fauna habitats on the island. A desktop review identified 135 fauna species that are reported to occur on Dauan. This can be compared with the 384 terrestrial fauna species that have been reported for the broader Torres Strait Island group. The Dauan fauna comprises 20 reptiles, 100 birds, 3 frogs and 12 mammals. Of these, one reptile, one bird and four mammal species are introduced. -

The Taxonomy, Chorology and Reproductive Biology of Southern Afri Can Meliaceae and Ptaeroxylaceae
Bothalia 16.2: 143-168 (1986) The taxonomy, chorology and reproductive biology of southern Afri can Meliaceae and Ptaeroxylaceae F. WHITE* Keywords: chorology. Meliaceae. Ptaeroxylaceae. reproductive biology, southern Africa, taxonomy ABSTRACT Information is provided on the taxonomy, chorology and reproductive biology of 14 indigenous and two intro duced species of Meliaceae in southern Africa, and on Ptaeroxylon (Ptaeroxylaceae). Two new taxa are described: Nymanieae F. White, tribus nov. and Turraea strevi F. White & B. T. Styles, sp. nov. Nurmonia (Harms) F. White, comb, et stat. nov.. a new section of Turraea L. is created. The account complements the treatments of these families in the Flora o f southern Africa. UITTREKSEL Inligting word verskaf oor die taksonomie. chorologie en voortplantingsbiologie van 14 inheemse en twee inge- voerde spesies van Meliaceae in suidelike Afrika en oor Ptaeroxylon (Ptaeroxylaceae). Twee nuwe taksons word beskryf: Nymanieae F. White, tribus nov. en Turraea strevi F. White & B. T. Styles, sp. nov. Nurmonia (Harms) F. White, comb, et stat. nov., 'n nuwe seksie van Turraea L. word geskep. Hierdie verslag is aanvullend tot die behandelings van hierdie families in die Flora o f southern Africa. CONTENTS The position of Ptaeroxylon and Nyma nia............................................................ 163 Introduction.................................................................143 South African Trichilia: chemistry and Generic and family delimitation..................... .......144 the taxonomist's e y e .......................... 163 The position of Ptaeroxylon.................................144 Conclusions................................................... 163 The position of N ym ania.....................................144 Taxonomy as a visual a rt.............................. 163 The circumscription of Turraea..........................145 The Meliaceae and the chorology of south Notes on individual genera and species ern Africa.................................................. 164 1. -

Bark Medicines Used in Traditional Healthcare in Kwazulu-Natal, South Africa: an Inventory
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector South African Journal of Botany 2003, 69(3): 301–363 Copyright © NISC Pty Ltd Printed in South Africa — All rights reserved SOUTH AFRICAN JOURNAL OF BOTANY ISSN 0254–6299 Bark medicines used in traditional healthcare in KwaZulu-Natal, South Africa: An inventory OM Grace1, HDV Prendergast2, AK Jäger3 and J van Staden1* 1 Research Centre for Plant Growth and Development, School of Botany and Zoology, University of Natal Pietermaritzburg, Private Bag X01, Scottsville 3209, South Africa 2 Centre for Economic Botany, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AE, United Kingdom 3 Department of Medicinal Chemistry, Royal Danish School of Pharmacy, 2 Universitetsparken, 2100 Copenhagen 0, Denmark * Corresponding author, e-mail: [email protected] Received 13 June 2002, accepted in revised form 14 March 2003 Bark is an important source of medicine in South Overlapping vernacular names recorded in the literature African traditional healthcare but is poorly documented. indicated that it may be unreliable in local plant identifi- From thorough surveys of the popular ethnobotanical cations. Most (43%) bark medicines were documented literature, and other less widely available sources, 174 for the treatment of internal ailments. Sixteen percent of species (spanning 108 genera and 50 families) used for species were classed in threatened conservation cate- their bark in KwaZulu-Natal, were inventoried. gories, but conservation and management data were Vernacular names, morphological and phytochemical limited or absent from a further 62%. There is a need for properties, usage and conservation data were captured research and specialist publications to address the in a database that aimed to synthesise published infor- gaps in existing knowledge of medicinal bark species mation of such species. -

Biodiversity Baseline in the Different Stages of the Project for the 10 Most Important Species
Drafted by: November 2018 Baseline report of amphibians, reptiles, and Haptanthus hazlettii Jilamito Hydroelectric Project Baseline study of amphibians, reptiles and the arboreal species Haptanthus hazlettii in the site of the Jilamito Hydroelectric Project FINAL REPORT Research team: Ricardo Matamoros (Main Coordinator) José Mario Solís Ramos (Herpetologist – Field Coordinator) Carlos M. O'Reilly (Botanist) Josué Ramos Galdámez (Herpetologist) Juan José Rodríguez (Field Technician) Dilma Daniela Rivera (Field Technician) Rony E. Valle (Field Technician) Technical support team and local guides: Hegel Velásquez (INGELSA Technician) - Forest Engineer Omar Escalante (INGELSA Environmental Technician) Nelson Serrano (ICF Tela Technician) Mauro Zavala (PROLANSATE Technician) Alberto Ramírez (Field Guide) José Efraín Sorto (Field Guide) Juan Ramírez (Field Guide) Agustín Sorto Natarén (Field Guide) Manuel Sorto Natarén (Field Guide) José Hernán Flores (Field Guide) Photos on the cover: The arboreal species, Haptanthus hazlettii, found in bloom. In the pictures we observe: Plectrohyla chrysopleura (Climbing frog), Atlantihyla spinipollex (Ceiba stream frog), Duellmanohyla salvavida (Honduran brook frog), Pleistioson sumichrastri (blue tail lizard), Bothriechis guifarroi (green Tamagas, palm viper). 2 Baseline report of amphibians, reptiles, and Haptanthus hazlettii Jilamito Hydroelectric Project 1 Content 2. SUMMARY ...................................................................................................................... 5 3. INTRODUCTION -
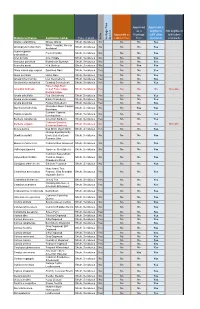
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

Phylogenetic Relationships of Ruteae (Rutaceae): New Evidence from the Chloroplast Genome and Comparisons with Non-Molecular Data
ARTICLE IN PRESS Molecular Phylogenetics and Evolution xxx (2008) xxx–xxx Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogenetic relationships of Ruteae (Rutaceae): New evidence from the chloroplast genome and comparisons with non-molecular data Gabriele Salvo a,*, Gianluigi Bacchetta b, Farrokh Ghahremaninejad c, Elena Conti a a Institute of Systematic Botany, University of Zürich, Zollikerstrasse 107, CH-8008 Zürich, Switzerland b Center for Conservation of Biodiversity (CCB), Department of Botany, University of Cagliari, Viale S. Ignazio da Laconi 13, 09123 Cagliari, Italy c Department of Biology, Tarbiat Moallem University, 49 Dr. Mofatteh Avenue, 15614 Tehran, Iran article info abstract Article history: Phylogenetic analyses of three cpDNA markers (matK, rpl16, and trnL–trnF) were performed to evaluate Received 12 December 2007 previous treatments of Ruteae based on morphology and phytochemistry that contradicted each other, Revised 14 July 2008 especially regarding the taxonomic status of Haplophyllum and Dictamnus. Trees derived from morpho- Accepted 9 September 2008 logical, phytochemical, and molecular datasets of Ruteae were then compared to look for possible pat- Available online xxxx terns of agreement among them. Furthermore, non-molecular characters were mapped on the molecular phylogeny to identify uniquely derived states and patterns of homoplasy in the morphological Keywords: and phytochemical datasets. The phylogenetic analyses determined that Haplophyllum and Ruta form Ruta reciprocally exclusive monophyletic groups and that Dictamnus is not closely related to the other genera Citrus family Morphology of Ruteae. The different types of datasets were partly incongruent with each other. The discordant phy- Phytochemistry logenetic patterns between the phytochemical and molecular trees might be best explained in terms of Congruence convergence in secondary chemical compounds. -

Synoptic Overview of Exotic Acacia, Senegalia and Vachellia (Caesalpinioideae, Mimosoid Clade, Fabaceae) in Egypt
plants Article Synoptic Overview of Exotic Acacia, Senegalia and Vachellia (Caesalpinioideae, Mimosoid Clade, Fabaceae) in Egypt Rania A. Hassan * and Rim S. Hamdy Botany and Microbiology Department, Faculty of Science, Cairo University, Giza 12613, Egypt; [email protected] * Correspondence: [email protected] Abstract: For the first time, an updated checklist of Acacia, Senegalia and Vachellia species in Egypt is provided, focusing on the exotic species. Taking into consideration the retypification of genus Acacia ratified at the Melbourne International Botanical Congress (IBC, 2011), a process of reclassification has taken place worldwide in recent years. The review of Acacia and its segregates in Egypt became necessary in light of the available information cited in classical works during the last century. In Egypt, various taxa formerly placed in Acacia s.l., have been transferred to Acacia s.s., Acaciella, Senegalia, Parasenegalia and Vachellia. The present study is a contribution towards clarifying the nomenclatural status of all recorded species of Acacia and its segregate genera. This study recorded 144 taxa (125 species and 19 infraspecific taxa). Only 14 taxa (four species and 10 infraspecific taxa) are indigenous to Egypt (included now under Senegalia and Vachellia). The other 130 taxa had been introduced to Egypt during the last century. Out of the 130 taxa, 79 taxa have been recorded in literature. The focus of this study is the remaining 51 exotic taxa that have been traced as living species in Egyptian gardens or as herbarium specimens in Egyptian herbaria. The studied exotic taxa are accommodated under Acacia s.s. (24 taxa), Senegalia (14 taxa) and Vachellia (13 taxa).