Spawning, Larval Rearing and Growth of the Silver Sillago in Abu Dhabi Omer M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VARIATION in SETTLEMENT and LARVAL DURATION of Klng GEORGE WHITING, SILLAGINODES PUNCTATA (SILLAGINIDAE), in SWAN BAY, VICTORIA, AUSTRALIA
BULLETIN OF MARINE SCIENCE, 54(1): 281-296, 1994 VARIATION IN SETTLEMENT AND LARVAL DURATION OF KlNG GEORGE WHITING, SILLAGINODES PUNCTATA (SILLAGINIDAE), IN SWAN BAY, VICTORIA, AUSTRALIA Gregory P. Jenkins and Helen M. A. May ABSTRACT Otoliths were examined from late-stage larvae and juveniles of King George whiting, Sillaginodes punctata, collected from Swan Bay in spring 1989. Increments in otoliths of larval S. punctata are known to be formed daily. A transition in the microstructure of otoliths from late-stage larvae was apparently related to environmental changes associated with entry to Port Phillip Bay. The pattern of abundance of post-larvae of S. punctata in fortnightly samples supported the contention that the transition was formed immediately prior to "set- tlement" in seagrass habitats. Backcalculation to the otolith transition suggested that five cohorts had entered Swan Bay, each approximately 10 days apart, from late September to early November. Stability of this pattern for juveniles from sequential samples indicated that otolith increments continued to be formed daily in the juvenile stage. The pattern of settlement was consistent for two sites within Swan Bay. The larval phase of King George whiting settling in Port Phillip Bay was extremely long and variable, ranging from approximately 100 to 170 days. Age at settlement was more variable than length, and growth rate at settlement was I I extremely slow, approximately 0.06 mm ·d- • • Backcalculated hatching dates ranged from April to JUly. July. Increment widths in the larval stage suggest that growth slows after approxi- mately 45 to 75 days; beyond which individuals are in a slow growth, competent stage of 40 to 100 days. -

Traditional Ecological Knowledge in Andavadoaka, Southwest Madagascar
BLUE VENTURES CONSERVATION REPORT Josephine M. Langley Vezo Knowledge: Traditional Ecological Knowledge in Andavadoaka, southwest Madagascar 52 Avenue Road, London N6 5DR [email protected] Tel: +44 (0) 208 341 9819 Fax: +44 (0) 208 341 4821 BLUE VENTURES CONSERVATION REPORT Andavadoaka, 2006 © Blue Ventures 2006 Copyright in this publication and in all text, data and images contained herein, except as otherwise indicated, rests with Blue Ventures. Recommended citation: Langley, J. (2006). Vezo Knowledge: Traditional Ecological Knowledge in Andavadoaka, southwest Madagascar. BLUE VENTURES CONSERVATION REPORT Summary Many fisheries and marine conservation management (ii) Knowledge of marine resources is passed orally from projects throughout the world have been dogged by generation to generation failure as a result of a lack of acceptance of (iii) Traditional laws, taboos and ceremonies are commonly management interventions by local communities. used in natural resource management Evaluation of these failed studies has produced (iv) Lifestyle, food security and housing are all dependent extensive guidelines, manuals and new fields of study on natural resources and the use of coastal and marine (Bunce and Pomeroy, 2004). Community engagement, resources form an essential part of this participatory research, and promoting the use of local (v) The arrival of the Catholic Mission has reduced the knowledge have repeatedly emerged as steps necessary proportion of villagers adhering to traditional ancestor to address the problem of managing the development of worship people and their economies while simultaneously (vi) There has been a change in recent years from a barter protecting the environment (Berkes et al, 2001; Bunce and subsistence economy to a market-driven cash- based economy and Pomeroy, 2004; Wibera et al, 2004; Scholz et al, (vii) Increased income for some members of the 2004). -

Marine Biodiversity of the South East NRM Region
Marine Environment and Ecology Benthic Ecology Subprogram Marine Biodiversity of the South East NRM Region SARDI Publication No. F2009/000681-1 SARDI Research Report series No. 416 Keith Rowling, Shirley Sorokin, Leonardo Mantilla and David Currie SARDI Aquatic Sciences PO BOX 120 Henley Beach SA 5022 December 2009 Prepared for the Department for Environment and Heritage 1 Information Systems and Database Support Program Marine Biodiversity of the South East NRM Region Keith Rowling, Shirley Sorokin, Leonardo Mantilla and David Currie December 2009 SARDI Publication No. F2009/000681-1 SARDI Research Report Series No. 416 Prepared for the Department for Environment and Heritage 2 This Publication may be cited as: Rowling, K.P., Sorokin, S.J., Mantilla, L. & Currie, D.R.. (2009) Marine Biodiversity of the South East NRM Region. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2009/000681-1. South Australian Research and Development Institute SARDI Aquatic Sciences 2 Hamra Avenue West Beach SA 5024 Telephone: (08) 8207 5400 Facsimile: (08) 8207 5406 http://www.sardi.sa.gov.au DISCLAIMER The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Chief of Division. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. SARDI does not accept any liability for the contents of this report or for any consequences arising from its use or any reliance placed upon it. -

Diversity and Length-Weight Relationships of Blenniid Species (Actinopterygii, Blenniidae) from Mediterranean Brackish Waters in Turkey
EISSN 2602-473X AQUATIC SCIENCES AND ENGINEERING Aquat Sci Eng 2019; 34(3): 96-102 • DOI: https://doi.org/10.26650/ASE2019573052 Research Article Diversity and Length-Weight relationships of Blenniid Species (Actinopterygii, Blenniidae) from Mediterranean Brackish Waters in Turkey Deniz İnnal1 Cite this article as: Innal, D. (2019). Diversity and length-weight relationships of Blenniid Species (Actinopterygii, Blenniidae) from Mediterranean Brackish Waters in Turkey. Aquatic Sciences and Engineering, 34(3), 96-102. ABSTRACT This study aims to determine the species composition and range of Mediterranean Blennies (Ac- tinopterygii, Blenniidae) occurring in river estuaries and lagoon systems of the Mediterranean coast of Turkey, and to characterise the length–weight relationship of the specimens. A total of 15 sites were surveyed from November 2014 to June 2017. A total of 210 individuals representing 3 fish species (Rusty blenny-Parablennius sanguinolentus, Freshwater blenny-Salaria fluviatilis and Peacock blenny-Salaria pavo) were sampled from five (Beşgöz Creek Estuary, Manavgat River Es- tuary, Karpuzçay Creek Estuary, Köyceğiz Lagoon Lake and Beymelek Lagoon Lake) of the locali- ties investigated. The high juvenile densities of S. fluviatilis in Karpuzçay Creek Estuary and P. sanguinolentus in Beşgöz Creek Estuary were observed. Various threat factors were observed in five different native habitats of Blenny species. The threats on the habitat and the population of the species include the introduction of exotic species, water ORCID IDs of the authors: pollution, and more importantly, the destruction of habitats. Five non-indigenous species (Prus- D.İ.: 0000-0002-1686-0959 sian carp-Carassius gibelio, Eastern mosquitofish-Gambusia holbrooki, Redbelly tilapia-Copt- 1Burdur Mehmet Akif Ersoy odon zillii, Stone moroko-Pseudorasbora parva and Rainbow trout-Oncorhynchus mykiss) were University, Department of Biology, observed in the sampling sites. -

Comprehensive Transcriptome Analysis Reveals Insights Into Phylogeny and Positively Selected Genes of Sillago Species
animals Article Comprehensive Transcriptome Analysis Reveals Insights into Phylogeny and Positively Selected Genes of Sillago Species Fangrui Lou 1, Yuan Zhang 2, Na Song 2, Dongping Ji 3 and Tianxiang Gao 1,* 1 Fishery College, Zhejiang Ocean University, Zhoushan 316022, Zhejiang, China; [email protected] 2 Fishery College, Ocean University of China, Qingdao 266003, Shandong, China; [email protected] (Y.Z.); [email protected] (N.S.) 3 Agricultural Machinery Service Center, Fangchenggang 538000, Guangxi, China; [email protected] * Correspondence: [email protected]; Tel.: +86-580-2089-333 Received: 24 February 2020; Accepted: 1 April 2020; Published: 7 April 2020 Simple Summary: A comprehensive transcriptome analysis revealed the phylogeny of seven Sillago species. Selection force analysis in seven Sillago species detected 44 genes positively selected relative to other Perciform fishes. The results of the present study can be used as a reference for the further adaptive evolution study of Sillago species. Abstract: Sillago species lives in the demersal environments and face multiple stressors, such as localized oxygen depletion, sulfide accumulation, and high turbidity. In this study, we performed transcriptome analyses of seven Sillago species to provide insights into the phylogeny and positively selected genes of this species. After de novo assembly, 82,024, 58,102, 63,807, 85,990, 102,185, 69,748, and 102,903 unigenes were generated from S. japonica, S. aeolus, S. sp.1, S. sihama, S. sp.2, S. parvisquamis, and S. sinica, respectively. Furthermore, 140 shared orthologous exon markers were identified and then applied to reconstruct the phylogenetic relationships of the seven Sillago species. The reconstructed phylogenetic structure was significantly congruent with the prevailing morphological and molecular biological view of Sillago species relationships. -

The Queensland Stout Whiting Fishery 1991 to 2002
FISHERY ASSESSMENT REPORT DPI AGENCY FOR FOOD AND FIBRE SCIENCES THE QUEENSLAND STOUT WHITING FISHERY 1991 TO 2002 Michael O’Neill 1 Kate Yeomans 2, Ian Breddin2, Eddie Jebreen2 and Adam Butcher1 1 AFFS FISHERIES – RESOURCE ASSESSMENT 2 QUEENSLAND FISHERIES SERVICE MARCH 2002 CONTENTS 1. EXECUTIVE SUMMARY ...............................................................................................................3 2. INTRODUCTION .............................................................................................................................4 2.1 MAIN FEATURES OF THE 2002 FISHERY........................................................................................4 2.2 HISTORY .......................................................................................................................................4 2.3 BIOLOGY.......................................................................................................................................5 2.4 MARKETING .................................................................................................................................6 2.5 MANAGEMENT..............................................................................................................................6 2.6 PREVIOUS ASSESSMENTS .............................................................................................................7 3. 2003 ASSESSMENT..........................................................................................................................8 3.1 ADVANCES -

Final Report
FINAL REPORT REHABILITATION AND CONSERVATION THE SEAGRASS MEADOWS AT CAM HAI DONG, CAM RANH BAY, KHANH HOA PROVINCE, CENTRAL VIETNAM. Pham Huu Tri Institute of Oceanography Nhatrang, Vietnam NHATRANG, JUNE 2008 Table of Contents 1. INTRODUCTION: 3 2. OBJECTIVES: 4 3. MATERIALS AND METHODS: 4 3.1 Research methods: 4 3.2 Project sites: 4 3. 3 Duration of the project: 6 3.4 Planting techniques: 6 3.4.1 Seagrasses: 6 3.4.2 Sea-horse and sea-cucumber: 7 3.4.3 Methods of study the leaf growth rate and leaf production: 7 3.4.4 Management and taking care of the rehabilitated area at Cam Hai Dong, Cam Ranh ba: 7 4. RESULTS: 7 4.1 The natural environmental elements in the rehabilitated area – Cam Hai Dong- Cam Ranh: 7 4.2 Species diversity of seagrasses in the rehabilitated area: 8 4.3 Transplantation the Enhalus acoroides for planting: 9 4.3.1 For the shoots method: 9 4.3.2 For the seedlings methods: 11 4. 4 Results of restoration the sea-horse at the rehabilitated area: 13 4.5 Results of restoration the sea-cucumber at the rehabilitated area: 14 5. CONCLUSION: 15 6. RECOMMENDATION: 15 LITERATURE CITED: 16 APPENDIX: 17 2 1. INTRODUCTION: Khanh Hoa is the coastal province in southern central Vietnam, situated between 11°45’02’’ to 13°02’ 10’’ North latitude and from 108045’10’’to 109045’06’’ East longitude, the length of the coast lines about 300km. The shallow coastal area of Khanh Hoa province is often covered by seagrass beds especially in Cam Ranh bay area where the seagrass beds have concentrated distribution with the superficies up to more than 1000 ha, there are 6 common species of seagrasses have been found there, among them Enhalus acoroides which has long leaves (1-2 m), dense density (40- 100 shoots/m2) and high cover (70-100%) is dominant. -

Information Sheet
Illustration © R. Swainston/anima.net.au Information sheet Yellowfin whiting (Sillago schombergkii) Distribution Identification Yellowfin whiting (also known as western sand Adults have no distinguishing body markings and are whiting) are endemic to south-western Australia from best identified by their yellow ventral and anal fins Western Australia (Onslow) to South Australia (Gulf St and a weakly forked tail. Juveniles have faint black Vincent). WA and SA host separate breeding stocks. blotches on the body and may be confused with juvenile western trumpeter whiting. Stock structure and movement In WA, populations within the Gascoyne Coast Growth Bioregion and West Coast Bioregion are believed Can reach a maximum of 427 mm total length (TL) to have limited connectivity and so are regarded as and 12 years of age. Females and males attain separate stocks. sexual maturity at 2 years, at 200 and 190 mm TL, respectively. Females grow slightly larger than males. In the West Coast Bioregion, some fish live in estuaries for much the year, but these fish migrate Reproduction to sea to join their ocean-dwelling counterparts to Spawning typically occurs at water temperatures spawn in late spring/early summer. Hence, fish of 22-24 ºC. Spawning occurs August-December across the West Coast Bioregion belong to the same in northern areas (e.g. Shark Bay) and December- breeding stock (e.g. fish caught in the Peel-Harvey February in southern areas (e.g. Perth). Spawning Estuary are part of the same population as those in occurs over a longer period in northern areas Geographe Bay or Jurien Bay). -

Proceedings of the American Lobster Transferable Trap Workshop
Special Report No. 75 of the Atlantic States Marine Fisheries Commission Proceedings of the American Lobster Transferable Trap Workshop October 2002 Proceedings of the American Lobster Transferable Trap Workshop October 2002 Edited by Heather M. Stirratt Atlantic States Marine Fisheries Commission Convened by: Atlantic States Marine Fisheries Commission August 26, 2002 Washington, DC Proceedings of the American Lobster Transferable Trap Workshop ii Preface This document was prepared in cooperation with the Atlantic States Marine Fisheries Commission’s American Lobster Management Board, Technical Committee, Plan Development Team, Stock Assessment Subcommittee and the Advisory Panel. A report of the Atlantic States Marine Fisheries Commission pursuant to U.S. Department of Commerce, National Oceanic and Atmospheric Administration Award No. NA17FG2205. Proceedings of the American Lobster Transferable Trap Workshop iii Acknowledgments This report is the result of a Workshop on transferable trap programs for American lobster which was held on August 26, 2002, in Washington, DC. The workshop was convened and organized by a Workshop Steering Committee composed of: David Spencer (Advisory Panel, Chair), George Doll (New York Commercial Lobsterman, Advisory Panel Member), John Sorlien (Rhode Island Commercial Lobsterman, Advisory Panel Member), Todd Jesse (Massachusetts Commercial Lobsterman, Advisory Panel Member), Ernie Beckwith (Connecticut Department of Environmental Protection), Mark Gibson (Rhode Island Department of Environmental Management), -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -
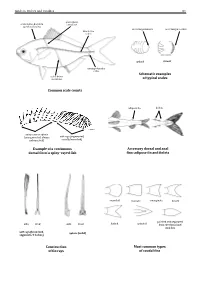
Field Identification Guide to the Living Marine Resources in Kenya
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

5-Review-Fish-Habita
United Nations UNEP/GEF South China Sea Global Environment Environment Programme Project Facility UNEP/GEF/SCS/RWG-F.8/5 Date: 12th October 2006 Original: English Eighth Meeting of the Regional Working Group for the Fisheries Component of the UNEP/GEF Project: “Reversing Environmental Degradation Trends in the South China Sea and Gulf of Thailand” Bangka Belitung Province, Indonesia 1st - 4th November 2006 INFORMATION COLLATED BY THE FISHERIES AND HABITAT COMPONENTS OF THE SOUTH CHINA SEA PROJECT ON SITES IMPORTANT TO THE LIFE- CYCLES OF SIGNIFICANT FISH SPECIES UNEP/GEF/SCS/RWG-F.8/5 Page 1 IDENTIFICATION OF FISHERIES REFUGIA IN THE GULF OF THAILAND It was discussed at the Sixth Meeting of the Regional Scientific and Technical Committee (RSTC) in December 2006 that the Regional Working Group on Fisheries should take the following two-track approach to the identification of fisheries refugia: 1. Review known spawning areas for pelagic and invertebrate species, with the aim of evaluating these sites as candidate spawning refugia. 2. Evaluate each of the project’s habitat demonstration sites as potential juvenile/pre-recruit refugia for significant demersal species. Rationale for the Two-Track Approach to the Identification of Fisheries Refugia The two main life history events for fished species are reproduction and recruitment. It was noted by the RSTC that both of these events involve movement between areas, and some species, often pelagic fishes, migrate to particular spawning areas. It was also noted that many species also utilise specific coastal habitats such as coral reefs, seagrass, and mangroves as nursery areas. In terms of the effects of fishing, most populations of fished species are particularly vulnerable to the impacts of high levels of fishing effort in areas and at times where there are high abundances of (a) stock in spawning condition, (b) juveniles and pre-recruits, or (c) pre-recruits migrating to fishing grounds.