A Review on in Situ Biodegradation of Methyl Parathion Through Soil Microbes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Insecticide Use for Vector-Borne Disease Control
WHO/CDS/NTD/WHOPES/GCDPP/2007.2 GLOBAL INSECTICIDE USE FOR VECTOR-BORNE DISEASE CONTROL M. Zaim & P. Jambulingam DEPARTMENT OF CONTROL OF NEGLECTED TROPICAL DISEASES (NTD) WHO PESTICIDE EVALUATION SCHEME (WHOPES) First edition, 2002 Second edition, 2004 Third edition, 2007 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication. CONTENTS Page Acknowledgements i Introduction 1 Collection of information 2 Data analysis and observations on reporting 3 All uses in vector control 6 Malaria vector control 22 Dengue vector control 38 Chagas disease vector control 48 Leishmaniasis vector control 52 Other vector-borne disease control 56 Selected insecticides – DDT 58 Selected insecticides – Insect growth regulators 60 Selected insecticides – Bacterial larvicides 62 Country examples 64 Annex 1. -
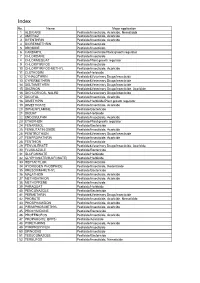
No. Name Major Application 1 ALDICARB Pesticide/Insecticide
Index No. Name Major application 1 ALDICARB Pesticide/Insecticide, Acaricide, Nematicide 2 AMITRAZ Pesticide/Insecticide, Acaricide 3 BIFENTHRIN Pesticide/Insecticide, Acaricide 4 BIORESMETHRIN Pesticide/Insecticide 5 BROMIDE Pesticide/Insecticide 6 CARBARYL Pesticide/Insecticide/Plant growth regulator 7 CHLORDANE Pesticide/Insecticide 8 CHLORMEQUAT Pesticide/Plant growth regulator 9 CHLORPYRIFOS Pesticide/Insecticide 10 CHLORPYRIFOS-METHYL Pesticide/Insecticide, Acaricide 11 CLETHODIM Pesticide/Herbicide 12 CYHALOTHRIN Pesticide&Veterinary Drugs/Insecticide 13 CYPERMETHRIN Pesticide&Veterinary Drugs/Insecticide 14 DELTAMETHRIN Pesticide&Veterinary Drugs/Insecticide 15 DIAZINON Pesticide&Veterinary Drugs/Insecticide, Acaricide 16 DICHLORVOS、NALED Pesticide&Veterinary Drugs/Insecticide 17 DICOFOL Pesticide/Insecticide, Acaricide 18 DIMETHIPIN Pesticide/Herbicide/Plant growth regulator 19 DIMETHOATE Pesticide/Insecticide, Acaricide 20 DIPHENYLAMINE Pesticide/Bactericide 21 DIQUAT Pesticide/Herbicide 22 ENDOSULFAN Pesticide/Insecticide, Acaricide 23 ETHEPHON Pesticide/Plant growth regulator 24 FENARIMOL Pesticide/Bactericide 25 FENBUTATIN OXIDE Pesticide/Insecticide, Acaricide 26 FENITROTHION Pesticide&Veterinary Drugs/Insecticide 27 FENPROPATHRIN Pesticide/Insecticide, Acaricide 28 FENTHION Pesticide/Insecticide 29 FENVALERATE Pesticide&Veterinary Drugs/Insecticide, Acaricide 30 FLUSILAZOLE Pesticide/Bactericide 31 GLUFOSINATE Pesticide/Herbicide 32 GLYPHOSATE(SULFOSATE) Pesticide/Herbicide 33 HEPTACHLOR Pesticide/Insecticide 34 HYDROGEN -

Dimethoate 4EC Systemic Insecticide – Miticide ACTIVE INGREDIENT: PRECAUTIONARY STATEMENTS (Cont.) Dimethoate*
GROUP 1B INSECTICIDE Dimethoate 4EC Systemic Insecticide – Miticide ACTIVE INGREDIENT: PRECAUTIONARY STATEMENTS (Cont.) Dimethoate* .......................................................... 43.5% Mixers, loaders, applicators, flaggers, and other handlers must OTHER INGREDIENTS: .......................................... 56.5% wear: Long-sleeved shirt and long pants, shoes plus socks, goggles TOTAL: .............................................................. 100.0% or face shield, chemical-resistant gloves, a NIOSH-approved * This product contains 4 pounds of dimethoate per gallon. dust/mist filtering respirator with MSHA/NIOSH approval number prefix TC-21C or a NIOSH-approved respirator with any N, R, P, or KEEP OUT OF REACH OF CHILDREN HE filter, and chemical-resistant apron when mixing, loading, clean- ing up spills, or equipment. WARNING See Engineering Controls for additional requirements and excep- Si usted no entiende la etiqueta, busque a alguien para que se la ex- tions. plique a usted en detalle. (If you do not understand the label, find Follow manufacturer’s instructions for cleaning/maintaining PPE. If someone to explain it to you in detail.) no such instructions for washables exist, use detergent and hot water. Keep and wash PPE separately from other laundry. See FIRST AID Below Discard clothing and other absorbent materials that have been EPA Reg. No. 19713-231 Net Content: drenched or heavily contaminated with this product’s concentrate. EPA Est. No. 19713-GA-001 2.5 Gals. (9.46 L) Do not reuse them. ENGINEERING CONTROLS FIRST AID Mixers and loaders supporting aerial application to alfalfa, cotton, IF SWALLOWED: soybeans, corn, safflower, sorghum, and wheat, must use a closed • Call a poison control center or doctor immediately for treatment system that meets the requirements listed in the Worker Protections advice. -

ACEPHATE (Addendum)
3 ACEPHATE (addendum) First draft prepared by Professor P.K. Gupta 1 and Dr Angelo Moretto 2 1 Rajinder Nagar, Bareilly, UP, India; 2 Dipartimento Medicina Ambientale e Sanità Pubblica, Università di Padova, Padova, Italy Explanation..........................................................................................................3 Evaluation for acceptable daily intake.................................................................4 Biochemical aspects ......................................................................................4 Oral absorption, distribution, excretion and metabolism .......................4 Toxicological studies.....................................................................................5 Acute toxicity.........................................................................................5 Short-term studies of toxicity.................................................................6 Special studies........................................................................................7 Studies on inhibition of cholinesterase activity in vitro ..................7 Short-term study of neurotoxicity ...................................................7 Developmental neurotoxicity..........................................................9 Observations in humans ..............................................................................10 Comments..........................................................................................................12 Toxicological evaluation ...................................................................................13 -

488 Subpart A—Organic Pesticide Chemicals Manufacturing
§ 455.11 40 CFR Ch. I (7–1–12 Edition) chemical products and be considered a this subpart are applicable to dis- ‘‘stand-alone’’ PFPR facility. charges resulting from the manufac- ture of the following organic active in- [43 FR 17776, Apr. 25, 1978, as amended at 50 FR 40701, Oct. 4, 1985; 51 FR 44911, Dec. 15, gredients: Aldrin, BHC, Captan, 1986; 58 FR 50689, Sept. 28, 1993; 61 FR 57548, Chlordane, DDD, DDE, DDT, Dichloran, Nov. 6, 1996] Dieldrin, Endosulfan, Endrin, Hepta- chlor, Lindane, Methoxychlor, Mirex, Subpart A—Organic Pesticide PCNB, Toxaphene, Trifluralin, Chemicals Manufacturing Azinphos Methyl, Demeton-O, Demeton-S, Diazinon, Disulfoton, Mal- Subcategory athion, Parathion Methyl, Parathion Ethyl, Aminocarb, Carbaryl, SOURCE: 43 FR 44846, Sept. 29, 1978, unless Methiocarb, Mexacarbate, Propoxur, otherwise noted. Barban, Chlorpropham, Diuron, Fenuron, Fenuron-TCA, Linuron, § 455.11 Compliance date for pretreatment standards for existing Monuron, Monuron-TCA, Neubron, sources (PSES). Propham, Swep, 2,4-D, Dicamba, Silvex, 2,4,5-T, Siduron, Perthane, and All discharges subject to Dicofol. pretreatment standards for existing (c) The intermediates used to manu- sources (PSES) in subparts A and B of facture the active ingredients and ac- this part must comply with the stand- tive ingredients used solely in experi- ards no later than September 28, 1993. mental pesticides are excluded from [61 FR 57549, Nov. 6, 1996] coverage in this subpart. Insecticidal pathogenic organisms such as Bacillus § 455.20 Applicability; description of thuringiensis, insect growth hormones, the organic pesticide chemicals plant extracts such as pyrethrins; sex manufacturing subcategory. attractants and botanicals such as Ro- (a) For the purpose of calculating and tenone are also excluded from BPT applying effluent limitations for COD, coverage in this subpart. -

Monocrotophos Proposed AEGL Document
MONOCROTOPHOS Page 1 of 32 Proposed 10/2009 v.1 1 2 3 4 ACUTE EXPOSURE GUIDELINE LEVELS 5 (AEGLs) 6 7 8 PROPOSED 9 10 11 12 13 MONOCROTOPHOS 14 (CAS Reg. No. 6923-22-4) 15 16 17 18 19 20 MONOCROTOPHOS Page 2 of 32 Proposed 10/2009 v.1 1 2 PREFACE 3 4 Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 5 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous 6 Substances (NAC/AEGL Committee) has been established to identify, review and interpret 7 relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic 8 chemicals. 9 10 AEGLs represent threshold exposure limits for the general public and are applicable to 11 emergency exposure periods ranging from 10 minutes to 8 hours. Three levels C AEGL-1, 12 AEGL-2 and AEGL-3 C are developed for each of five exposure periods (10 and 30 minutes, 1 13 hour, 4 hours, and 8 hours) and are distinguished by varying degree of severity of toxic effects. 14 The three AEGLs are defined as follows: 15 16 AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per 17 cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general 18 population, including susceptible individuals, could experience notable discomfort, irritation, or 19 certain asymptomatic, non-sensory effects. However, the effects are not disabling and are 20 transient and reversible upon cessation of exposure. -

Downloads/Pgm Hydrus1d/HYDRUS-4.16.Pdf (Accessed on 20 April 2019)
water Article Assessment of the Environmental Risk of Pesticides Leaching at the Watershed Scale under Arid Climatic Conditions and Low Recharge Rates Hesham M. Ibrahim 1,2,* and Ali M. Al-Turki 1 1 Department of Soil Science, College of Food and Agricultural Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia; [email protected] 2 Department of Soils and Water, Faculty of Agriculture, Suez Canal University, Ismailia 41522, Egypt * Correspondence: [email protected]; Tel.: +966-501728656 Received: 5 January 2020; Accepted: 2 February 2020; Published: 5 February 2020 Abstract: The assessment of the vulnerability of soil and groundwater resources to pesticide contamination is important to reduce the risk of environmental pollution. The applicability of the expanded attenuation factor (EAF) to assess leaching potential of 30 pesticides was investigated under 1 four recharge rates (0.0003–0.002 m d− ) in the arid environment of the Jazan watershed. EAF results revealed that Picloram, Carbofuran, Monocrotophos, and 2,4-D pesticides showed high leaching potential, mainly because of their low KOC, and relatively longer t1/2. In addition, medium leaching potential was observed with six more pesticides (Atrazine, Aldicarb, Simazine, Methomyl, Oxamyl, and Lindane). Regardless of the recharge rate, all other pesticides showed a very low leaching potential in the Jazan watershed. Sensitivity analysis revealed that the output of the EAF index is most sensitive to the fraction of organic carbon ( foc), water content at field capacity (θFC ), recharge rate (q), and partition coefficient (KOC), and least sensitive to soil bulk density (ρb) and air-filled porosity (na). -

Florida State Emergency Response Commission
Florida State Emergency Response Commission Sub-Committee on Training (SOT) HAZARDOUS MATERIALS MEDICAL TREATMENT PROTOCOLS Version 3.3 TOXIDROMES Toxidromes are clinical syndromes that the patient presents with. These patterns of signs and symptoms are essential for the successful recognition of chemical exposure. The toxidromes identified in this protocol are chemical exposure based while others such as the opioids are found within general medical protocol. These chemical toxidromes are identified clinically into five syndromes: Irritant Gas Toxidrome Asphyxiant Toxidrome Corrosive Toxidrome Hydrocarbon and Halogenated Hydrocarbons Toxidrome Cholinergic Toxidrome Each can present as a clinical manifestation of the chemical/poisoning involved with some cross-over between toxidromes. This list combines the toxic syndromes found within NFPA 473 (A.5.4.1(2) and traditional syndromes. Toxidrome Correlation to NFPA Standard 473 and Traditional Syndromes Toxidrome NFPA 473 A.5.4.1(2) Hazardous Materials Protocol Correlation Irritant Gas (j) Irritants Bronchospasm OC Pepper spray & lacrimants Asphyxiant (c) Chemical asphyxiants Carbon Monoxide (d) Simple asphyxiants Aniline dyes, Nitriles, Nitrares (h) Blood Agents Cyanide & Hydrogen Sulfide (n) Nitrogen Compounds Closed Space Fires Simple Asphyxants Corrosive (a) Corrosives Hydrofluroic Acid (g) Vesicants Chemical burns to the eye Choramine and Chlorine Hydrocarbon (e) Organic solvents Phenol and (q) Phenolic Compounds Halogenated Hydrocarbons Halogenated Hydrocarbons Cholinergic (b) Pesticides -

The Spruce Budworm, Choristoneura Fumiferana
02-01370 Spruce Budworm Bro 10/10/02 11:09 AM Page 1 MORE INFORMATION The he spruce budworm, Choristoneura fumiferana For more information on Spruce Budworms call: The Tree Line Spruce (Clemens), is the most destructive and widely (204) 945-7866. Or write: Budworm distributed forest defoliator in North America. Manitoba Conservation Forestry Branch In Manitoba T Forest Health and Ecology The destructive phase of this pest is the larval or caterpillar 200 Saulteaux Crescent Winnipeg, Manitoba R3J 3W3 stage. Massive budworm outbreaks occur periodically, Web site: www.gov.mb.ca/natres/forestry/ destroying hundreds of thousands of hectares of valuable fir and spruce. Aerial view of budworm damage In eastern Canada the budworm’s preferred food is balsam fir, Photos courtesy of Canadian Forest Service, Great Lakes Forest Research Centre, white spruce and red spruce. In Manitoba, the budworm Sault Ste. Marie, Ontario and Northern Forest Research Centre, Edmonton, Alberta. feeds primarily on white spruce and balsam fir, and, less frequently, on black spruce. 02-01370 Spruce Budworm Bro 10/10/02 11:09 AM Page 2 DESCRIPTION OF LIFE STAGES LIFE CYCLE DAMAGE CONTROL The adult moth has a wingspread of The female moth lays In light and moderate infestations Various insecticides are used 21 to 30 mm. It is grey-brown in its eggs in July on the damage is restricted to a partial against the spruce budworm to colour with silvery white patches on underside of needles. loss of new foliage, particularly in protect valuable spruce and fir the forewings. Normally, the eggs the upper crown trees. -

For Methyl Parathion
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES MEMORANDUM DATE: July 31, 2006 SUBJECT: Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides FROM: Debra Edwards, Director Special Review and Reregistration Division Office of Pesticide Programs TO: Jim Jones, Director Office of Pesticide Programs As you know, EPA has completed its assessment of the cumulative risks from the organophosphate (OP) class of pesticides as required by the Food Quality Protection Act of 1996. In addition, the individual OPs have also been subject to review through the individual- chemical review process. The Agency’s review of individual OPs has resulted in the issuance of Interim Reregistration Eligibility Decisions (IREDs) for 22 OPs, interim Tolerance Reassessment and Risk Management Decisions (TREDs) for 8 OPs, and a Reregistration Eligibility Decision (RED) for one OP, malathion.1 These 31 OPs are listed in Appendix A. EPA has concluded, after completing its assessment of the cumulative risks associated with exposures to all of the OPs, that: (1) the pesticides covered by the IREDs that were pending the results of the OP cumulative assessment (listed in Attachment A) are indeed eligible for reregistration; and 1 Malathion is included in the OP cumulative assessment. However, the Agency has issued a RED for malathion, rather than an IRED, because the decision was signed on the same day as the completion of the OP cumulative assessment. -

Organophosphate Poisoning : a Review
120 Sinha and Sharma Med J Indones Organophosphate poisoning : A review Parmod K. Sinha, Ashok Sharma Abstrak Pestisida organofosfat digunakan secara luas di seluruh dunia. Keracunan oleh bahan ini merupakan masalah kesehatan masyarakat, terutama di negara berkembang. Zat neurotoksik organofosfat merupakan bahan yang dianggap mengancam dalam bidang militer dan terorisme. Mekanisme toksisitas bahan ini adalah dengan cara menghambat asetilkolinesterase yang mengakibatkan menumpuknya neurotransmitor asetilkolin dan terjadi rangsangan terus-menerus pada reseptor asetilkolin pada sistem saraf sentral maupun perifer. Selain krisis kolinergik, organofosfat dapat menimbulkan berbagai sindrom neurologis, baik akut maupun kronik. Sedangkan gejala peralihan ( intermediate) terjadi 1-4 hari setelah krisis kolinergik teratasi. Pengobatan standar terdiri dari reaktivasi asetilkolinesterase dengan antidot golongan oksim (prolidoksim, oksidoksime, HI-6 dan HLo7), dan pengendalian efek biokimia asetilkolin dengan menggunakan atropin. Golongan oksim yang baru HI-6 dan Hlo7 merupakan reaktivator asetilkolinesterase yang lebih cocok dan efektif untuk keracunan akut dan berat dibandingkan dengan prolidoksim dan obidoksim. Penderita yang mendapat pengobatan segera, biasanya dapat sembuh dari toksisitas akut, namun gejala neurologis ikutan dapat saja terjadi. (Med J Indones 2003; 12: 120-6) Abstract Organophosphate pesticides are used extensively worldwide, and poisoning by these agents, particularly in developing nations is a public health problem. Organophosphorous -

Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2
Technical Publication 91-01 Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2 by Richard J. Pfeuffer January 1991 This publication was produced at an annual cost of $243.75 or $.49 per copy to inform the public. 500 191 Produced on recycled paper. Water Quality Division Research and Evaluation Department South Florida Water Management District West Palm Beach, Florida A IBSTRAC'I' Pesticide monitoring data are collected under the requirements of several permits and agreements as an indicator of water quality. The monitoring provides data to determine shifts or adverse trends in the quality of water being delivered to Lake Okeechobee, Everglades National Park, and the Water Conservation Areas. In addition, pesticide residue data are collected throughout the South Florida Water Management District at locations selected to determine water quality conditions at the major water control points. Special investigations are performed on selected pesticides as required and follow-up sampling is conducted based on the pesticides detected. Data were collected from 13 stations in 1984. By 1988, the network was expanded to 29 stations. Currently, water and sediment samples are collected quarterly and analyzed for 67 pesticides, herbicides and degradation products. Out of a total of 197 surface water samples, 13 percent had detectable residues, while 25 percent of the 208 sediment samples had detectable residues. The compounds detected in the water samples included atrazine and zinc phosphide while a variety of compounds, including DDT, have been detected in the sediment. None of the residues detected are considered to have adverse health or environmental effects.