Monitoring of Breathing Activity Using Smartphone-Acquired Signals Bersain A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Emergencies in Paediatrics and Neonatology Published and Forthcoming Titles in the Emergencies in … Series
OXFORD MEDICAL PUBLICATIONS Emergencies in Paediatrics and Neonatology Published and forthcoming titles in the Emergencies in … series: Emergencies in Adult Nursing Edited by Philip Downing Emergencies in Anaesthesia Edited by Keith Allman, Andrew McIndoe, and Iain H. Wilson Emergencies in Cardiology Edited by Saul G. Myerson, Robin P. Choudhury, and Andrew Mitchell Emergencies in Children’s and Young People’s Nursing Edited by E.A. Glasper, Gill McEwing, and Jim Richardson Emergencies in Clinical Surgery Edited by Chris Callaghan, Chris Watson and Andrew Bradley Emergencies in Critical Care, 2e Edited by Martin Beed, Richard Sherman, and Ravi Mahajan Emergencies in Gastroenterology and Hepatology Marcus Harbord and Daniel Marks Emergencies in Mental Health Nursing Edited by Patrick Callaghan Emergencies in Obstetrics and Gynaecology Edited by S. Arulkumaran Emergencies in Oncology Edited by Martin Scott-Brown, Roy A.J. Spence, and Patrick G. Johnston Emergencies in Paediatrics and Neonatology, 2e Edited by Stuart Crisp and Jo Rainbow Emergencies in Palliative and Supportive Care Edited by David Currow and Katherine Clark Emergencies in Primary Care Chantal Simon, Karen O’Reilly, John Buckmaster, and Robin Proctor Emergencies in Psychiatry, 2e Basant Puri and Ian Treasaden Emergencies in Radiology Edited by Richard Graham and Ferdia Gallagher Emergencies in Respiratory Medicine Edited by Robert Parker, Catherine Thomas, and Lesley Bennett Emergencies in Sports Medicine Edited by Julian Redhead and Jonathan Gordon Head, Neck and Dental -

Sectional Survey of Staff Physicians, Residents and Medical Students
Open access Original research BMJ Open: first published as 10.1136/bmjopen-2020-044240 on 26 March 2021. Downloaded from Influence of language skills on the choice of terms used to describe lung sounds in a language other than English: a cross-sectional survey of staff physicians, residents and medical students Abraham Bohadana , Hava Azulai, Amir Jarjoui, George Kalak, Ariel Rokach, Gabriel Izbicki To cite: Bohadana A, Azulai H, ABSTRACT Strengths and limitations of this study Jarjoui A, et al. Influence of Introduction The value of chest auscultation would be language skills on the choice enhanced by the use of a standardised terminology. To ► To our knowledge, this is the first study to examine of terms used to describe lung that end, the recommended English terminology must sounds in a language other than the transfer to language other than English of the be transferred to a language other than English (LOTE) English: a cross-sectional survey recommended lung sound terminology in English. without distortion. of staff physicians, residents and ► True sound classification was validated by computer- Objective To examine the transfer to Hebrew—taken as medical students. BMJ Open based sound analysis. 2021;11:e044240. doi:10.1136/ a model of LOTE—of the recommended terminology in ► Participants were from the same hospital—which English. bmjopen-2020-044240 tends to limit the study generalisability—but had Design/setting Cross- sectional study; university- based Prepublication history and different clinical and educational background. ► hospital. supplemental material for this ► Use of more complex sounds (eg, rhonchus and Participants 143 caregivers, including 31 staff paper is available online. -
![The Health Bulletin [Serial]](https://docslib.b-cdn.net/cover/9544/the-health-bulletin-serial-869544.webp)
The Health Bulletin [Serial]
HEALTH SCIENCES LIBRARY OF THE UNIVERSITY OF NORTH CAROLINA This book must not be token from the Library building. Form No. 471 NOTICE TO READER.— When you finish reading this magazine place a one-cent stamp on this notice, hand same to »ny postal em- ployee and it will be placed in the hands of our soldiers or sailors atihe front. NO WRAPPING— NO ADDRESS. Thl5 BulkliAwillbe 5er\t free to qimj citizen of the State uporxreguest j as second-clasa Entered matter at Postoffice at Raleigh, N. C, under Act of July 16, 1894. Published monthly at the office ef the Secretary of the Board, Raleigh, N. O. Vol..XXXIII APRIL, 1918 No. 1 HOGS OR FOLKS, WHICH? seepages ONLY THE PEOPLE CAN LOOSE THE BONDS TABLE OF CONTENTS Hogs or Folks, Which ? 3 Physician Found Guilty 13 Sentekced to Prison oe Steriliza- More Intelligent Excitement tion 3 Needed 13 Soldiers and Tobacco 4 Paste This on Your Mirror 14 Popular Mistakes 4 What Vaccination Will Do 14 State Death Rate 5 Low Know How to Live 15 Three Things to Do 5 Spring Fever and Bran ... 16 Tanlac—The Master Medicine .... 5 Wheat Large Scars and Sore Arms Unneces- First Aid Instructions 17 sary 6 How to Stop Worrying 18 Cancer Not Inherited 7 Gasoline as an Emergency Medicine 19 School Epidemics 8 Don't Stand so Much 19 Where Ignorance is Crimin.a.l 9 Safe Guide to Healthful Eating. 19 Play is the Thing 10 Saving Mothers 20 How an Epidemic Developed 11 Why Register a Baby? 21 Sex Hygiene 12 Avoid Early Handicaps 21 Typhoid Bacillus Carries foe Over Why Nurse Your Baby ? 21 Forty Years 12 Have Early Diagnosis 22 Open-Air Schools 12 Symptoms of Tuberculosis 24 MEMBERS OF THE NORTH CAROLINA STATE BOARD OF HEALTH J. -

Investigation of Respiratory Disease Chapter 4.2 (B)
4.1 THE CLINICAL PRESENTATION OF CHEST DISEASES 347 Table 1 Modified Borg Scale∗ Chapter 4.1 Number Verbal description 10 Severe The clinical presentation of chest 9 diseases 8 Moderately severe 7 D. J. Lane 6 5 Moderate 4 The predominant symptoms of chest diseases are cough, breath- 3 lessness, chest pain and haemoptysis. 2 Slight 1 0 None ∗Modified from Borg, G.A.V. (1982). Psychological basis of perceived exertion. Cough Medical Science of Sports and Exercise, 14, 377–81. The cough reflex is initiated by stimulation of receptors in the larynx and major airways, by mechanical or chemical irritants. The afferent fibres run in branches of the superior laryngeal nerve and vagus. Haemoptysis A dry cough, short and repeated, is heard in tracheobronchitis A definite cause is only found in some 50 per cent of cases and it is and early pneumonia. In laryngitis the sound is hoarse and harsh. important to be sure that the blood does truly come from the lungs In abductor paralysis of the vocal cords it is prolonged and and not from the nose or gastrointestinal tract. Haemoptysis is a blowing. Weakness of thoracic muscles lessens the expulsive force classical presenting feature of tuberculosis, carcinoma, and bronchi- and cough may be suppressed when there is severe thoracic or ectasis, but there are many other causes, for instance Goodpasture’s upper abdominal pain. Cough with expectoration in the morning syndrome, mitral valve disease, coagulation defects, or even endo- is characteristic of chronic bronchitis and large volumes of yellow metriosis. It is rare in pulmonary embolism, when it reflects infarction sputum throughout the day suggests bronchiectasis. -

The Complete Poetry of James Hearst
The Complete Poetry of James Hearst THE COMPLETE POETRY OF JAMES HEARST Edited by Scott Cawelti Foreword by Nancy Price university of iowa press iowa city University of Iowa Press, Iowa City 52242 Copyright ᭧ 2001 by the University of Iowa Press All rights reserved Printed in the United States of America Design by Sara T. Sauers http://www.uiowa.edu/ϳuipress No part of this book may be reproduced or used in any form or by any means without permission in writing from the publisher. All reasonable steps have been taken to contact copyright holders of material used in this book. The publisher would be pleased to make suitable arrangements with any whom it has not been possible to reach. The publication of this book was generously supported by the University of Iowa Foundation, the College of Humanities and Fine Arts at the University of Northern Iowa, Dr. and Mrs. James McCutcheon, Norman Swanson, and the family of Dr. Robert J. Ward. Permission to print James Hearst’s poetry has been granted by the University of Northern Iowa Foundation, which owns the copyrights to Hearst’s work. Art on page iii by Gary Kelley Printed on acid-free paper Library of Congress Cataloging-in-Publication Data Hearst, James, 1900–1983. [Poems] The complete poetry of James Hearst / edited by Scott Cawelti; foreword by Nancy Price. p. cm. Includes index. isbn 0-87745-756-5 (cloth), isbn 0-87745-757-3 (pbk.) I. Cawelti, G. Scott. II. Title. ps3515.e146 a17 2001 811Ј.52—dc21 00-066997 01 02 03 04 05 c 54321 01 02 03 04 05 p 54321 CONTENTS An Introduction to James Hearst by Nancy Price xxix Editor’s Preface xxxiii A journeyman takes what the journey will bring. -

Automatic Adventitious Respiratory Sound Analysis: a Systematic Review
RESEARCH ARTICLE Automatic adventitious respiratory sound analysis: A systematic review Renard Xaviero Adhi Pramono, Stuart Bowyer, Esther Rodriguez-Villegas* Department of Electrical and Electronic Engineering, Imperial College London, London, United Kingdom * [email protected] Abstract a1111111111 Background a1111111111 Automatic detection or classification of adventitious sounds is useful to assist physicians in a1111111111 a1111111111 diagnosing or monitoring diseases such as asthma, Chronic Obstructive Pulmonary Dis- a1111111111 ease (COPD), and pneumonia. While computerised respiratory sound analysis, specifically for the detection or classification of adventitious sounds, has recently been the focus of an increasing number of studies, a standardised approach and comparison has not been well established. OPEN ACCESS Citation: Pramono RXA, Bowyer S, Rodriguez- Objective Villegas E (2017) Automatic adventitious respiratory sound analysis: A systematic review. To provide a review of existing algorithms for the detection or classification of adventitious PLoS ONE 12(5): e0177926. https://doi.org/ respiratory sounds. This systematic review provides a complete summary of methods used 10.1371/journal.pone.0177926 in the literature to give a baseline for future works. Editor: Thomas Penzel, Charite - UniversitaÈtsmedizin Berlin, GERMANY Received: December 16, 2016 Data sources Accepted: May 5, 2017 A systematic review of English articles published between 1938 and 2016, searched using Published: May 26, 2017 the Scopus (1938-2016) -

Understanding Lung Sounds, Third Edi- Structive Pulmonary Disease to Oxygen Ther- Fectious Processes, and the List of Infectious Tion
BOOKS,FILMS,TAPES,&SOFTWARE tion in the text. The editors used art spar- material. I found that the book is supportive style of a traditional textbook. The reader ingly and wisely, where needed; for of the current National Institutes of Health can pause and formulate his or her own an- example, flow volume tracings and other recommendations for treating acute respira- swers before proceeding to the text’s an- graphics to illustrate pulmonary functions. tory distress syndrome. I was also encour- swers. In practice it is easy to disseminate The illustrations will greatly enhance the aged to see a discussion on multiple-organ the required information, which adds to this reader’s understanding, and there are excel- dysfunction syndrome, as well as informa- text’s utility as a reference. The design of lent illustrations in many chapters, such as tion on risk factors, morbidity, and mortal- the text stimulates the evaluation of a prob- the chapters “Mediastinoscopy” and “Gen- ity. Another nice facet of this book is its lem and the formulation of creative, effec- eral Approaches to Interstitial Lung Dis- discussions of current controversies in acute tive solutions for patient care. Teaching crit- ease.” The radiographs and computed to- respiratory distress syndrome management. ical thinking in this way creates better mography images, though not abundant, In the section on mechanical ventilation clinicians, which benefits our patients. adequately demonstrate specific and impor- there is an informative discussion on the Overall, Pulmonary/Respiratory Ther- tant clinical findings. Image quality is im- basics of mechanical ventilation, as well as apy Secrets is informative, enlightening, portant to illustrate points effectively, and I an interesting discussion on the mechanisms and interesting. -

Towards the Standardisation of Lung Sound Nomenclature
TASK FORCE REPORT ERS STATEMENT Towards the standardisation of lung sound nomenclature Hans Pasterkamp1, Paul L.P. Brand2,3, Mark Everard4, Luis Garcia-Marcos5,6, Hasse Melbye7 and Kostas N. Priftis8 Affiliations: 1Section of Respirology, Dept of Pediatrics and Child Health, University of Manitoba, Winnipeg, MB, Canada. 2Princess Amalia Children’s Center, Isala Hospital, Zwolle, The Netherlands. 3Postgraduate School of Medicine, University Medical Centre and University of Groningen, Groningen, The Netherlands. 4School of Paediatrics, University of Western Australia, Princess Margaret Hospital, Subiaco, Australia. 5Pediatric Respiratory and Allergy Units, Arrixaca University Children’s Hospital, University of Murcia, Murcia, Spain. 6IMIB-Arrixaca Biohealth Research Institute, Murcia, Spain. 7General Practice Research Unit, Faculty of Health Sciences, UIT the Arctic University of Norway, Tromsø, Norway. 8Children’s Respiratory and Allergy Unit, Third Dept of Paediatrics, “Attikon” Hospital, University of Athens Medical School, Athens, Greece. Correspondence: Hans Pasterkamp, Winnipeg Children’s Hospital, CS516 – 840 Sherbrook St, Winnipeg, Manitoba, R3A 1S1, Canada. E-mail: [email protected] ABSTRACT Auscultation of the lung remains an essential part of physical examination even though its limitations, particularly with regard to communicating subjective findings, are well recognised. The European Respiratory Society (ERS) Task Force on Respiratory Sounds was established to build a reference collection of audiovisual recordings of lung sounds that should aid in the standardisation of nomenclature. Five centres contributed recordings from paediatric and adult subjects. Based on pre-defined quality criteria, 20 of these recordings were selected to form the initial reference collection. All recordings were assessed by six observers and their agreement on classification, using currently recommended nomenclature, was noted for each case. -

Audio-Visual Recognition of Auscultatory Breathing Sounds Using Fourier and Wavelet Analyses
Asian Journal of Computer and Information Systems (ISSN: 2321 – 5658) Volume 03– Issue 04, October 2015 Audio-visual Recognition of Auscultatory Breathing Sounds using Fourier and Wavelet Analyses 1* 2 2 3 4 Fumio Nogata , Yasunai Yokota , Yoko Kawamura , Hiroyuki Morita and Yoshiyuki Uno 1 Emeritus Professor, Gifu University 2 Department of Electrical, Electronic and Computer Engineering 501-1193, 1-1Yanagido Gifu (Japan) 3Graduate School of Medicine, Gifu University 501-1193, 1-1Yanagido Gifu (Japan) 4Gifu Prefectural General Medical Center 500-8717, 4-6-1 Noishiki, Gifu (Japan) *Corresponding author’s email: nogata [AT] gifu-u.ac.jp ABSTRACT— The era of computer management of clinical data demands the establishment of new techniques to analyze auscultatory sounds that can be better understood by both physicians and patients. This paper describes visual-based recognition techniques of breath sounds using two spectrograms created using short term FFT and wavelet analyses. Changes of frequency, intensity, and tone with time of breath sounds (21 samples) were shown using spectrograms of two kinds. Consequently, abnormal breath sounds were simply detected by differences of those patterns at first sight. They assist the recognition of the associated condition of disease. We expect to become a diagnostic support system in the near future. Keywords— Breath sound, Visualization, Image analysis, STFFT, Wavelet 1. INTRODUCTION The invention of the stethoscope by René Laennec supported auscultation, a practical new method of bedside examination [1]. Auscultation is performed to examine the circulatory, respiratory, and gastrointestinal systems. However, this subjective method depends on long clinical experience, good listening skills, and auditory perception to differentiate sound patterns. -

The Respiratory Sound Features of COVID-19 Patients Fill Gaps Between Clinical Data and Screening Methods
medRxiv preprint doi: https://doi.org/10.1101/2020.04.07.20051060; this version posted April 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. The respiratory sound features of COVID-19 patients fill gaps between clinical data and screening methods Yinghui Huang1, Sijun Meng2, Yi Zhang3, Shuisheng Wu4, Yu Zhang5, Yawei Zhang2, Yixiang Ye1, Qifeng Wei1, Niangui Zhao6, Jianping Jiang3, Xiaoying Ji2, Chunxia Zhou2, Chao Zheng7, Wen Zhang8, Lizhong Xie3, Yongchao Hu3, Jianquan He9, Jian Chen9, Wangyue Wang5, Liming Cao5, Wen Xu4, Yunhong Lei10, Zhenghua Jiang1, Weiping Hu7, Wenjuan Qin9, Wanyu Wang7, Yulong He2, Hang Xiao11, Xiaofang Zheng3, Yiqun Hu9, Wensheng Pan5, Changhua Zhang2, Jianfeng Cai1 1. First Hospital of Nanping, Nanping, Fujian Province, P. R. China, 314500 2.The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, Guangdong Province, P. R. China, 518107. 3. Pucheng County Hospital of Traditional Chinese Medicine, Pucheng, Fujian Province, P. R. China, 353400. 4. Fujian University of Traditional Chinese Medicine, No.1 of Qiuyang Road, Shangjie University Town, Fuzhou, Fujian Province, P.R. China, 350122. 5. Zhejiang provincial people's hospital, People's Hospital of Hangzhou Medical College, Hangzhou, Zhejiang Province, P. R. China, 310014. 6. The Second Afficiated Hospital of Xiamen Medical College, No.566 of Shengguang Road, Jimei District, Xiamen, Fujian Province, P. R. China, 361021 7. The First Affiliated Hospital of XiaMen University, Xiamen, 361003, Fujian Province, China. 8. -

Presentation of Pneumonia
7 Presentation of Pneumonia MONROE KARETZKY Clinical Presentation Dyspnea The sensation of shortness of breath-dyspnea, or breathlessness-cha racterized by tachypnea and variable degrees of hypoxemia and hypo capnea is a characteristic clinical phenomenon of pneumonia and occurs even in the absence of pleuritic pain or fever. The alveolar hyperventila tion reflected by the hypocapnia has been found to persist in patients with lobar pneumonia even after arterial hypoxemia is eliminated with oxygen therapy. A process of elimination can be observed in the long-standing efforts to attribute the sensation of breathlessness to objective criteria. Classic breath-holding studies have shown dyspnea to be alleviated by a rebreathing maneuver or with inspiration of oxygen and carbon dioxide gas mixtures that resulted in no improvement in either arterial hypoxemia or the associated respiratory acidosis. Thus linkage to chemoreceptor activity has been largely unsuccessful. Almost all of the afferent impulses arising from airway and intra pulmonary receptors are carried in the vagus nerves. Classic studies in dogs have reported that the tachypnea of lobar pneumonia was entirely dependent on intact vagi. Subsequently, stretch receptors of vagal afferents were shown to be of significance but not critical. More recent studies with differential block suggest that the lung receptors that are excited are those innervated by nonmyelinated vagal afferents.l These J receptors lie in the interstitial space of the lung at the alveolar level. The inflammatory mediators presumably act to depolarize these chemically sensitive non-myelinated nerve endings. It has been suggested that J-receptor stimulation alone or in conjunction with the stimulation of irritant receptors is responsible for the tachypnea of pneumonia. -
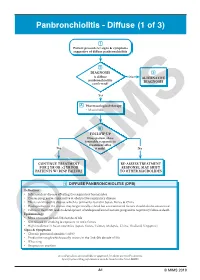
Panbronchiolitis - Diffuse (1 of 3)
Panbronchiolitis - Diffuse (1 of 3) 1 Patient presents w/ signs & symptoms suggestive of diffuse panbronchiolitis 2 DIAGNOSIS 3 Is diff use No ALTERNATIVE panbronchiolitis DIAGNOSIS confi rmed? Yes A Pharmacological therapy • Macrolides B FOLLOWUP Does patient show favorable response to treatment after Yes 6 mth? No CONTINUE TREATMENT REASSESS TREATMENT FOR 2 YR OR >2 YR FOR RESPONSE, MAY SHIFT PATIENTS W/ RESP FAILURE TO OTHER MACROLIDES 1 DIFFUSE PANBRONCHIOLITIS (DPB) Defi nition • Infl ammatory disease aff ecting the respiratory bronchioles • Causes progressive suppurative & obstructive respiratory disease • is is an idiopathic disease which is primarily found in Japan, Korea & China • Predisposition to the disease may be genetically-related but environmental factors should also be considered • Failure to treat DPB leads to development of widespread bronchiectasis, progressive respiratory failure & death Epidemiology • More common in 2nd-5th decade of life • Not related to smoking & exposure to toxic fumes • High incidence in Asian countries (Japan, Korea, Taiwan, Malaysia, China, ailand, Singapore) Signs & Symptoms • Chronic paranasal sinusitis (>80%) • Productive cough which usually occurs in the 2nd-5th decade of life • Wheezing© MIMS • Dyspnea on exertion Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. A1 © MIMS 2019 Panbronchiolitis - Diffuse (2 of 3) 2 DIAGNOSIS Physical Exam • Crackles, wheezing on chest exam Pulmonary Functions Tests • Spirometry, lung volume determination - May show signifi cant obstructive defect w/ or w/o superimposed restriction - Signifi cantly limited airways resistant to bronchodilators - FEV1/FVC <70%, vital capacity (VC) <80%, residual volume (RV) >150% - Hypoxemia may be present in blood gas analysis Panbronchiolitis - Diffuse Panbronchiolitis • P.