The Picking Table Volume 20, No. 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sarkinite Mn (Aso4)(OH)
2+ Sarkinite Mn2 (AsO4)(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Crystals typically thick tabular {100}, elongated, to 4 mm, short prismatic, or tabular along [010]. May be crudely spherical, granular massive. Physical Properties: Cleavage: On {100}, distinct. Fracture: Subconchoidal to uneven. Hardness = 4–5 D(meas.) = 4.08–4.18 D(calc.) = 4.20 Optical Properties: Semitransparent. Color: Flesh-red to dark blood-red, rose-red, orange, orange-brown, brown, reddish yellow to yellow; pale rose to yellow in transmitted light. Streak: Rose-red to yellow. Luster: Greasy. Optical Class: Biaxial (–). Pleochroism: Weak. Orientation: Y = b; X ∧ c = –54◦. Dispersion: r< v. Absorption: X > Z > Y. α = 1.790–1.793 β = 1.794–1.807 γ = 1.798–1.809 2V(meas.) = 83◦ Cell Data: Space Group: P 21/a. a = 12.779(2) b = 13.596(2) c = 10.208(2) β = 108◦530 Z=16 X-ray Powder Pattern: Pajsberg [Harstigen mine, near Persberg], Sweden. 3.18 (10), 3.04 (10), 3.29 (9), 3.48 (8), 2.90 (7), 2.65 (6), 6.0 (3) Chemistry: (1) (2) (3) (1) (2) (3) P2O5 0.21 ZnO 0.15 As2O5 41.60 44.09 43.23 PbO 0.25 CO2 0.76 MgO 0.98 0.19 FeO 0.13 0.02 CaO 1.40 0.29 MnO 51.60 51.77 53.38 H2O 3.06 [3.40] 3.39 CuO 0.01 insol. 0.38 Total 100.37 [99.92] 100.00 (1) Pajsberg [Harstigen mine, near Persberg], Sweden. -

NEW MINERAL NAMES Mrcnnnr Fr-Brscnpn
THE AMERICAN MINERAI,OGIST, VOL. 55, JANUARY-FEBRUARY, 1970 NEW MINERAL NAMES Mrcnnnr Fr-Brscnpn Barringerite P. R. Busrcr (1969) Phosphide from meteorites.' Barringerite, a new iron-nickel mineral. Sci,ence165, 169-17 1,. The average of microprobe analyses was Fe 44.3*0.9, Ni 33.9+0.7, Co 0.25+0.03, P 21.8+0.4, stm 10O.25/6,corresponding to (Irer.roNio.srCoo.n)P,or (Fe, Ni)rP. X-ray study shows it to be hexagonal,space group P62 m, a 5.87 -t0.07, c 3.M+0.04 ft. The strongest X-ray lines (including many overlapping troilite or schreibersite; those starred do not overlap) are 2.98 (110),2.85* (101),2.53(200),2.23(lll),2.03*(201), 1.88* (r20), r.72(t00), 1.68(300, t2r), L.48(220), t.4t(3t0, 22r), r.29*(31r), 1.28(122), t.27 (400), 1.205(302),1.197(4oD.The structure is similar to those of synthetic FerP and NirP. p (calc) 6.92. Color white, very similar to that of kamacite, bluish compared to schreibersite. Harder than either kamacite or schreibersite. Reflectivity in air and oil slightly higher than that of schreibersite, lower than that of kamacite. Noticeably anisotropic (white to blue). Bireflectance not observed. The mineral occurs as bands 10-15 pm wide and several hundred microns long; they consist of individual grains less than 1 pm in diameter. They occur in the Ollague pallasite along the contacts between schreibersite and troilite. The name is for D. -
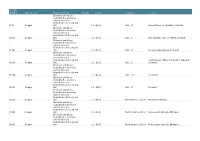
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments
minerals Article Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments Gabriel Ziwa 1,2,*, Rich Crane 1,2 and Karen A. Hudson-Edwards 1,2 1 Environment and Sustainability Institute, University of Exeter, Penryn TR10 9FE, UK; [email protected] (R.C.); [email protected] (K.A.H.-E.) 2 Camborne School of Mines, University of Exeter, Penryn TR10 9FE, UK * Correspondence: [email protected] Abstract: Cobalt is recognised by the European Commission as a “Critical Raw Material” due to its irreplaceable functionality in many types of modern technology, combined with its current high-risk status associated with its supply. Despite such importance, there remain major knowledge gaps with regard to the geochemistry, mineralogy, and microbiology of cobalt-bearing environments, particu- larly those associated with ore deposits and subsequent mining operations. In such environments, high concentrations of Co (up to 34,400 mg/L in mine water, 14,165 mg/kg in tailings, 21,134 mg/kg in soils, and 18,434 mg/kg in stream sediments) have been documented. Co is contained in ore and mine waste in a wide variety of primary (e.g., cobaltite, carrolite, and erythrite) and secondary (e.g., erythrite, heterogenite) minerals. When exposed to low pH conditions, a number of such minerals are 2+ known to undergo dissolution, typically forming Co (aq). At circumneutral pH, such aqueous Co can then become immobilised by co-precipitation and/or sorption onto Fe and Mn(oxyhydr)oxides. This paper brings together contemporary knowledge on such Co cycling across different mining environments. -

Summary Report
5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 SUMMARY REPORT OF THE GEOLOGICAL SURVEY DEPARTMENT OF CANADA F OR THE CALENDA R YEAR 1905 P R INTED BY OR DER OF P ARLIAME NT OTTAWA PRINTED BYS. E. DAWSON, PRIN'fER TO THE KING'S MOST EXCELLENT MAJESTY 1906 (No. 26-1906.J . .... ...... • -, . .. : : : ... ·: .. : ... ~ .. ...... : ... : : ., ; : : : .·. : ·. : ..- ·.. :····"·... : : ) · ~ .··· ·,·/ "• ..... ·.· : .. · : : :·· ·... .. ."' II.. ·. · :; ,.· •••· : ... • • ••··. , ".•:'"·.·:· "'.: . .. • : ·. : ••:: · ,:. • • • : : : . ·=· .... ...... ·. : :· .. ..... .. "., .: .~: . .. .. ... ~ " .... ... : : .. : : .. : ; : .. ' ~ ..... ...... ·.. ···.. : ...·" ·:·: .. ·... • .. .- .. ... .. : ·.· ..: ....·. ··. .. :; ·.·.:·.... ..... : ·. ...· .. ::·.: ... ......... ·:·• . • 5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 To His Excellency the Right Honourable Sir Albert Henry George, Earl Grey, ,Viscount Howick, Baron Grey of Howick, a Baronet, G. C. M. G., &:c., &:c., &:c., Governor General oj Canada. MAY IT PLEASE YOUR EXCELLENCY,- The undersigned has the honour to lay before Your Excellency, in compliance with 3 Vic., Chap. 2, Section 6, the Summary Report of the Operations of the Geological Survey Department for the calendar year ending December 31, 1905. Respectfully submitted. FRANK OLIVER, Ministe1· of the Interior . .. 5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 rrABLE 0 F CONTENTS SUMMARY R EPOR'l' OJI 1'ffll ACTING D mECTOR :- Advantage of Geological Surveys .. ..... 1 Geological Society of America . .............. .... 2 International -

Ore Bin / Oregon Geology Magazine / Journal
.TATE 0 .. O"EGON DUMTtIC..,. , O~ O.~"' ••I .. CIUJ. INDUS""". P'CNtTL.UIO. ORIOON THE ORE.-BIN VOL. ~ NO. , 12 PORTLAND, OREGON Permiuioa is ennttd to rc:print informatton C:Ol'ltain~ herdn. AAy crmit eiwn the Orcaon State I}q)artment of Gmlou' and Mineral Indldtric. for c:ompilinl this inf'ormMJon will be appreciated. vol. t, no . 12 THE ORI.-BIN D..... b.r 1942 Portland, Oregon STATE DEPARTIIENT OF GEOLOGY A IIlIIERAL INDUSTRIES Head Ofti •• : 702 'oodlark Bldg., Portland, Or.go.n State Governing Board Earl K. Nixon Director •• H. Strayer, Ohair.an, Baker Y. If. Libboy Kinins £ns1near Albert Burch lIodford John Eliot Allen Goologist E. B. II&.Naughton Portland H. C. Harrison Spe.tro •• op1st State A8say Laborator1e. 400 i. I Street, Grant. Pass 2102 Court Street, Baker Ray C. fr,.sher Field Geologist Noroan S. 'iagner Field Geologist Robart G. Bassett Assayer Hugh K. Lancaster AS80.yer COCKEYEDEAS Our pre.ise may b. sImply stated: ••••••• 11, never mind t he premise. That can come later. Let'e get on with the dlecu8slon. Con8Ider the followlng: We have been keeping records, 1n WashIngton and elsewhere, for the last 150 years, on almost every conceivable th1n, froa the price of dried apples to the trend in halitosls among rata. From records, graphs are made, • or Can be, If anyone is sufficiently curious. Graphs are wonderful. One oan prove almo.t anything by them. After one haa proved .omething, the chances are good that aomeone else may come along and, using another set of oft1cial records, draw a graph tha.t make. -

Redetermination of Eveite, Mn2aso4 (OH), Based on Single-Crystal X-Ray
inorganic compounds Acta Crystallographica Section E Experimental Structure Reports Crystal data Online ˚ 3 Mn2AsO4(OH) V = 469.33 (15) A ISSN 1600-5368 Mr = 265.81 Z =4 Orthorhombic, Pnnm Mo K radiation a = 8.5478 (16) A˚ = 12.29 mmÀ1 Redetermination of eveite, b = 8.7207 (16) A˚ T = 293 K c = 6.2961 (12) A˚ 0.05 Â 0.05 Â 0.04 mm Mn2AsO4(OH), based on single-crystal X-ray diffraction data Data collection Bruker APEXII CCD area-detector 3315 measured reflections diffractometer 911 independent reflections a b c Yongbo W. Yang, * Ryan A. Stevenson, Alesha M. Siegel Absorption correction: multi-scan 849 reflections with I >2(I) and Gordon W. Downsd (SADABS; Sheldrick, 2005) Rint = 0.015 Tmin = 0.579, Tmax = 0.639 aDepartment of Chemistry and Biochemistry, University of Arizona, 1306 Refinement E. University Blvd, Tucson, Arizona 85721-0041, USA, bDepartment of 2 2 Geosciences, University of Arizona, 1040 E. 4th Street, Tucson, Arizona R[F >2(F )] = 0.021 49 parameters 2 85721-0077, USA, cDepartment of Molecular and Cellular Biology, University of wR(F ) = 0.055 All H-atom parameters refined ˚ À3 Arizona, 1007 E. Lowell Street, Tucson, Arizona 85721-0106, USA, and dUniversity S = 1.09 Ámax = 1.28 e A 911 reflections Á = À1.15 e A˚ À3 High School, 421 N. Arcadia Avenue, Tucson, Arizona 85711-3032, USA min Correspondence e-mail: [email protected] Received 16 October 2011; accepted 24 October 2011 Table 1 Hydrogen-bond geometry (A˚ , ). Key indicators: single-crystal X-ray study; T = 293 K; mean (As–O) = 0.002 A˚; R factor = 0.021; wR factor = 0.055; data-to-parameter ratio = 18.6. -

54 SKUTTERUDITE FROX{ COBALT. ONTARIOI on Some Specimens
54 THE AMENICAN M]NENALOGIST SKUTTERUDITE FROX{ COBALT. ONTARIOI T. I/. WALKER Uruiuersily oJ Torontn On somespecimens recently obtained from the Temiskaming mine, Cobalt, Ontario, small brilliant crystals resembling smal- tite were observed,imbedded in fragments of soft chloritic or micaceouscountry rock includedin the vein. As is well known, smaltite and chloanthite closelyresemble one another and are commonlyintergrown in the samecrystal. It was with a view to determiningthe relative purity of the supposedsmaltite that this examinationwas undertaken. The principal ore in the vein is smaltite,usually massive, but occasionallyforming rough crystalsup to b or 6 millimeters in diameter. The rest of the vein matter is either calcite or lrag- ments of country rock. The small crystals under investigation seemto representthe latest mineral to form;they are tirr white, and verSzlustrous. The specific gravity, determined on 0.62 gram of carefully selectedcrystals, is 6.29,which is closerto the value usually given for skutterudite (CoAss) than to that for smaltite (CoAsz). The crystals, which seldom exceed a millimeter in diameter, were measured on a Goldschmidt goniometer. The fol- lowing forms were obseryed on each crystal: a(IOO), o(111), d(110), and n(211). The relative development of the forms is shown in figure 1. Nothing observed suggests that the crystals are Frc.1. hemihedral-the faces of the last two formswere not alwayspresent in full, but the omissionsappeared to be quite irregular. These four forms have all been observed on both smaltite and skutterudite. An analysis made on the powdered mineral is shown in I. l Presented at the firrt annual meeting of the Mineralogical Society of America, December 28, 1920. -

Metals from Ores: an Introduction
CRIMSONpublishers http://www.crimsonpublishers.com Mini Review Aspects Min Miner Sci ISSN 2578-0255 Metals from Ores: An Introduction Fathi Habashi* Department of Mining, Laval University, Canada *Corresponding author: Fathi Habashi, Department of Mining, Metallurgical and Materials Engineering, Laval University, Quebec City, Canada Submission: October 09, 2017; Published: December 11, 2017 Introduction of metallic lustre. Of these about 300 are used industrially in the chemical industry, in building materials, in fertilizers, as fuels, etc., chemical composition, constant physical properties, and a A mineral is a naturally occurring substance having a definite characteristic crystalline form. Ores are a mixture of minerals: they are processed to yield an industrial mineral or treated chemically and are known as the industrial minerals Figure 3. to yield a single or several metals. Ores that are generally processed for only a single metal are those of iron, aluminium, chromium, tin, mercury, manganese, tungsten, and some ores of copper. Gold ores may yield only gold, but silver is a common associate. Nickel ores are always associated with cobalt, while lead and zinc always occur together in ores. All other ores are complex yielding a number of metals. before being treated by chemical methods to recover the metals. Ores undergo a beneficiation process by physical methods and grinding then separation of the individual mineral by physical Figure 2: Metals and metalloids obtained from ores. Beneficiation processes involve liberation of minerals by crushing methods (gravity, magnetic, etc.) or physicochemical methods pyrometallurgical, and electrochemical methods. Metals and (flotation) Figure 1. Chemical methods involve hydrometallurgical, metalloids obtained from ores are shown in Figure 2. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

A Specific Gravity Index for Minerats
A SPECIFICGRAVITY INDEX FOR MINERATS c. A. MURSKyI ern R. M. THOMPSON, Un'fuersityof Bri.ti,sh Col,umb,in,Voncouver, Canad,a This work was undertaken in order to provide a practical, and as far as possible,a complete list of specific gravities of minerals. An accurate speciflc cravity determination can usually be made quickly and this information when combined with other physical properties commonly leads to rapid mineral identification. Early complete but now outdated specific gravity lists are those of Miers given in his mineralogy textbook (1902),and Spencer(M,i,n. Mag.,2!, pp. 382-865,I}ZZ). A more recent list by Hurlbut (Dana's Manuatr of M,i,neral,ogy,LgE2) is incomplete and others are limited to rock forming minerals,Trdger (Tabel,l,enntr-optischen Best'i,mmungd,er geste,i,nsb.ildend,en M,ineral,e, 1952) and Morey (Encycto- ped,iaof Cherni,cal,Technol,ogy, Vol. 12, 19b4). In his mineral identification tables, smith (rd,entifi,cati,onand. qual,itatioe cherai,cal,anal,ys'i,s of mineral,s,second edition, New york, 19bB) groups minerals on the basis of specificgravity but in each of the twelve groups the minerals are listed in order of decreasinghardness. The present work should not be regarded as an index of all known minerals as the specificgravities of many minerals are unknown or known only approximately and are omitted from the current list. The list, in order of increasing specific gravity, includes all minerals without regard to other physical properties or to chemical composition. The designation I or II after the name indicates that the mineral falls in the classesof minerals describedin Dana Systemof M'ineralogyEdition 7, volume I (Native elements, sulphides, oxides, etc.) or II (Halides, carbonates, etc.) (L944 and 1951). -

The Crystal Structure of the Mineral Scholzite and a Study of the Crystal
\ I 7'1,71 ¡1 :), THE CRYSTAL STRUCTURE OF THE MINERAL SCHOLZITE AND A STUDY OF THE CRYSTAL CHEMISTRY OF SOME RELATED PHOSPHATE AND ARSENATE MINERALS A thesis submitted for the Degree of Doctor of PhilosoPhY at the University of Adelaide in April, 1975 by R0DERICK JEFFREY HILL, B.Sc. (Hons.) Department of Geology and Mineralogy Au/¿tr¡'t ,,! /'/,".'','-'"' ' TABLE OF CONTENTS Page SUMMARY (i) STATEMENT OF ORIGINATITY (ii) ACKNOWLEDGEMENTS (iii) GENERAL INTRODUCTION I CHAPTER 1 TIIE GEOI.OGY AbID MINERALOGY OF REAPHOOK HILL, SOUTTI AUSTR.ALIA 2 1.1 ABSTR,ACT 2 r.2 INTRODUCTTON 2 1.3 GEOIÐGICAL SETTING 3 I.4 EXPERTMENTAI TECHNIQT ES 5 1.5 THE MAJOR PHOSPHATE MTNERALS 6 1.5.1 Tarbuttite - Znr(po4) (OH) 6 1.5.2 Parahopeite ZnrZn(pOn) - Z.4HZo 9 I.5.3 Scholzite CaZnU(pO4) - 2.2H2O 9 1.5.4 Zincian Collinsite Car(Mg,Zn) (pO4) - 2.2H2O 15 1.6 ASPECTS OF EHE CRYSTA¡ CHEMISTRY OF THE MAJOR PHOSPHATE MINERALS 19 L.7 PARAGENESIS 23 1.7.1 Major Minerals 23 L.7.2 Other lvlinerals 26 1.7.3 Conclusions 27 CHAPTER 2 TIIE CRYSTAI STRUCTURE OF SCHOLZITE 30 2.L ABSTRACT 30 2.2 INTRODUCTION 32 2.3 DATA COLLECTION AND ÐATA REDUCTION 32 2.4 DISCUSSION OF TITE INTENSITY DISTRIBUTION 35 2.4.L Subcell Structure and pseudosynunetry 35 2.4.2 Dete::mination of the True Syrnmetry 4L 2.4.3 Structural Disorder 4l 2.5 STRUqrURE SOLUTION AND REFINEMENT OF THE AVER.AGE ST'BCELL 52 2.6 DESCRIPTION AND DISCUSSION OF THE AVERAGE ST]BCELL STRUCTURE 60 2.6.I Topology 60 2.6.2 Disorder 65 2.6.3'iThermal" parameters 67 2.7 STRUCTURE SOLUTION AND REFTNEMENT OF TITE },IAIN CELL