Practical Geology and Mineralogy for Prospectors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Silver Sulphosalts in Galena from Espeland, Norway
Silver sulphosalts in galena from Espeland, Norway M. S. NAIK Naik, M. S.: Silver sulphosalts in galena from Espeland, Norway. Norsk Geo logisk Tidsskrift, Vol. 55, pp. 185-189. Oslo 1975. The silver-bearing phases in galena from Espeland are freibergite, unknown 'phase C' (PbuA&l!Sbto�), pyrargyrite, stephanite and hessite. The composi tions of these phases were determined by electron microprobe. Freibergite is homogeneous in composition with an average 35 percent silver. The average calculated atomic proportions (Cu,Ag,Fe)t2.�b4S12·4• deviate from the stoi chiometric composition (Cu,Ag,Fe,Znh2(SbAs)4S13 of tetrahedrite. It has been found that most of the silver present in the ore occurs in the silver phases and not in structural positions in the galena lattice. M. S. Naik, Mineralogisk-geologisk museum, Sars gate l, Oslo 5, Norway. Present address: lndian School of Mines, Dhanbad, Bihar, India. Galenas with high contents of bismuth and silver have been described from Espeland and other localities in Norway by Oftedal (1942). He proposed that Bi atoms enter into the PbS lattice without causing any distortion, if an equal number of Ag atoms enter simultaneously. The excess of Bi over (BiAg) was considered to be responsible for the octahedral parting in some of these galenas. In the present study of the silver bearing galena from Espeland it has been found that the galena contains inclusions of several silver-bearing phases, as well as native bismuth, while the galena itself contains only nor mal amounts of Ag and essentially no Bi. Geology of the Espeland deposit The Espeland deposit is located SW of Vegårshei church, between the farms Myre and Espeland in Aust-Agder fylke, southern Norway. -

Quantitative Ore Mineralogy and Variations in Vein Textures in the World-Class Waihi Epithermal Au-Ag District, New Zealand
©2016 Society of Economic Geologists, Inc. SEG-MJD 2016 Conference Quantitative Ore Mineralogy and Variations in Vein Textures in the World-Class Waihi Epithermal Au-Ag District, New Zealand Jeffrey L. Mauk,1,* Sarah J. Fyfe,2 Erin G. Skinner,3 Andrew H. Menzies,4 Heather Lowers,1 and Alan Koenig1 1U.S. Geological Survey, Denver, Colorado, USA 2University of Auckland, School of Environment, Auckland, New Zealand 3Pattle Delamore Partners Ltd., Auckland, New Zealand 4Universidad Católica del Norte, Facultad Ingeniería y Ciencias Geologicas, Antofagasta, Chile *Corresponding author: e-mail, [email protected] The Waihi district in the Hauraki Goldfield of New Zealand contains a series of adularia- sericite epithermal Au-Ag veins that has produced more than 6.8 Moz Au. We used reflected light microscopy, scanning electron microscopy, microprobe analyses, laser ablation- inductively coupled plasma-mass spectrometry (LA-ICP-MS), and automated mineralogy (QEMSCAN) to analyze ore mineralogy. We used hand sample examination, transmitted light microscopy, scanning electron microscopy, and scanning electron microscopy- cathodoluminescence (SEM-CL) to describe and characterize quartz textures in veins. Critical comparison of opaque minerals and quartz textures within eight major veins of the Waihi district shows significant mineralogical and textural variations among veins. The peripheral veins of the district (Martha, Favona, Moonlight, Cowshed, and Silverton) contain abundant colloform, cherty, and black quartz fill textures, with minor crustiform and massive quartz. The central veins (Amaranth, Trio, and Union) contain predominantly massive and crustiform textures, and these veins are also commonly coarser grained than peripheral veins. Most of the textures identified with SEM-CL correlate with textures that are visible in hand sample and under the microscope, and are well described in the epithermal literature, such as colloform bands, comb quartz, and moss texture. -

Minerals of the San Luis Valley and Adjacent Areas of Colorado Charles F
New Mexico Geological Society Downloaded from: http://nmgs.nmt.edu/publications/guidebooks/22 Minerals of the San Luis Valley and adjacent areas of Colorado Charles F. Bauer, 1971, pp. 231-234 in: San Luis Basin (Colorado), James, H. L.; [ed.], New Mexico Geological Society 22nd Annual Fall Field Conference Guidebook, 340 p. This is one of many related papers that were included in the 1971 NMGS Fall Field Conference Guidebook. Annual NMGS Fall Field Conference Guidebooks Every fall since 1950, the New Mexico Geological Society (NMGS) has held an annual Fall Field Conference that explores some region of New Mexico (or surrounding states). Always well attended, these conferences provide a guidebook to participants. Besides detailed road logs, the guidebooks contain many well written, edited, and peer-reviewed geoscience papers. These books have set the national standard for geologic guidebooks and are an essential geologic reference for anyone working in or around New Mexico. Free Downloads NMGS has decided to make peer-reviewed papers from our Fall Field Conference guidebooks available for free download. Non-members will have access to guidebook papers two years after publication. Members have access to all papers. This is in keeping with our mission of promoting interest, research, and cooperation regarding geology in New Mexico. However, guidebook sales represent a significant proportion of our operating budget. Therefore, only research papers are available for download. Road logs, mini-papers, maps, stratigraphic charts, and other selected content are available only in the printed guidebooks. Copyright Information Publications of the New Mexico Geological Society, printed and electronic, are protected by the copyright laws of the United States. -
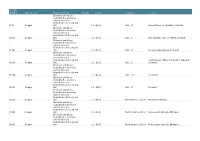
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments
minerals Article Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments Gabriel Ziwa 1,2,*, Rich Crane 1,2 and Karen A. Hudson-Edwards 1,2 1 Environment and Sustainability Institute, University of Exeter, Penryn TR10 9FE, UK; [email protected] (R.C.); [email protected] (K.A.H.-E.) 2 Camborne School of Mines, University of Exeter, Penryn TR10 9FE, UK * Correspondence: [email protected] Abstract: Cobalt is recognised by the European Commission as a “Critical Raw Material” due to its irreplaceable functionality in many types of modern technology, combined with its current high-risk status associated with its supply. Despite such importance, there remain major knowledge gaps with regard to the geochemistry, mineralogy, and microbiology of cobalt-bearing environments, particu- larly those associated with ore deposits and subsequent mining operations. In such environments, high concentrations of Co (up to 34,400 mg/L in mine water, 14,165 mg/kg in tailings, 21,134 mg/kg in soils, and 18,434 mg/kg in stream sediments) have been documented. Co is contained in ore and mine waste in a wide variety of primary (e.g., cobaltite, carrolite, and erythrite) and secondary (e.g., erythrite, heterogenite) minerals. When exposed to low pH conditions, a number of such minerals are 2+ known to undergo dissolution, typically forming Co (aq). At circumneutral pH, such aqueous Co can then become immobilised by co-precipitation and/or sorption onto Fe and Mn(oxyhydr)oxides. This paper brings together contemporary knowledge on such Co cycling across different mining environments. -

The Gersdorffite-Bismuthinite-Native Gold Association and the Skarn
minerals Article The Gersdorffite-Bismuthinite-Native Gold Association and the Skarn-Porphyry Mineralization in the Kamariza Mining District, Lavrion, Greece † Panagiotis Voudouris 1,* , Constantinos Mavrogonatos 1 , Branko Rieck 2, Uwe Kolitsch 2,3, Paul G. Spry 4 , Christophe Scheffer 5, Alexandre Tarantola 6 , Olivier Vanderhaeghe 7, Emmanouil Galanos 1, Vasilios Melfos 8 , Stefanos Zaimis 9, Konstantinos Soukis 1 and Adonis Photiades 10 1 Department of Geology & Geoenvironment, National and Kapodistrian University of Athens, 15784 Athens, Greece; [email protected] (C.M.); [email protected] (E.G.); [email protected] (K.S.) 2 Institut für Mineralogie und Kristallographie, Universität Wien, 1090 Wien, Austria; [email protected] 3 Mineralogisch-Petrographische Abteilung, Naturhistorisches Museum, 1010 Wien, Austria; [email protected] 4 Department of Geological and Atmospheric Sciences, Iowa State University, Ames, IA 50011, USA; [email protected] 5 Département de Géologie et de Génie Géologique, Université Laval, Québec, QC G1V 0A6, Canada; [email protected] 6 Université de Lorraine, CNRS, GeoRessources UMR 7359, Faculté des Sciences et Technologies, F-54506 Vandoeuvre-lès-Nancy, France; [email protected] 7 Université de Toulouse, Géosciences Environnement Toulouse (GET), UMR 5563 CNRS, F-31400 Toulouse, France; [email protected] 8 Department of Mineralogy-Petrology-Economic Geology, Faculty of Geology, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] 9 Institut für Mineralogie, TU Bergakademie Freiberg, 09599 Freiberg, Germany; [email protected] 10 Institute of Geology and Mineral Exploration (I.G.M.E.), 13677 Acharnae, Greece; [email protected] * Correspondence: [email protected]; Tel.: +30-210-7274129 † The paper is an extended version of our paper published in 1st International Electronic Conference on Mineral Science. -

Summary Report
5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 SUMMARY REPORT OF THE GEOLOGICAL SURVEY DEPARTMENT OF CANADA F OR THE CALENDA R YEAR 1905 P R INTED BY OR DER OF P ARLIAME NT OTTAWA PRINTED BYS. E. DAWSON, PRIN'fER TO THE KING'S MOST EXCELLENT MAJESTY 1906 (No. 26-1906.J . .... ...... • -, . .. : : : ... ·: .. : ... ~ .. ...... : ... : : ., ; : : : .·. : ·. : ..- ·.. :····"·... : : ) · ~ .··· ·,·/ "• ..... ·.· : .. · : : :·· ·... .. ."' II.. ·. · :; ,.· •••· : ... • • ••··. , ".•:'"·.·:· "'.: . .. • : ·. : ••:: · ,:. • • • : : : . ·=· .... ...... ·. : :· .. ..... .. "., .: .~: . .. .. ... ~ " .... ... : : .. : : .. : ; : .. ' ~ ..... ...... ·.. ···.. : ...·" ·:·: .. ·... • .. .- .. ... .. : ·.· ..: ....·. ··. .. :; ·.·.:·.... ..... : ·. ...· .. ::·.: ... ......... ·:·• . • 5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 To His Excellency the Right Honourable Sir Albert Henry George, Earl Grey, ,Viscount Howick, Baron Grey of Howick, a Baronet, G. C. M. G., &:c., &:c., &:c., Governor General oj Canada. MAY IT PLEASE YOUR EXCELLENCY,- The undersigned has the honour to lay before Your Excellency, in compliance with 3 Vic., Chap. 2, Section 6, the Summary Report of the Operations of the Geological Survey Department for the calendar year ending December 31, 1905. Respectfully submitted. FRANK OLIVER, Ministe1· of the Interior . .. 5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 rrABLE 0 F CONTENTS SUMMARY R EPOR'l' OJI 1'ffll ACTING D mECTOR :- Advantage of Geological Surveys .. ..... 1 Geological Society of America . .............. .... 2 International -

Acanthite Ag2s C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Monoclinic, Pseudo-Orthorhombic
Acanthite Ag2S c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic, pseudo-orthorhombic. Point Group: 2/m. Primary crystals are rare, prismatic to long prismatic, elongated along [001], to 2.5 cm, may be tubular; massive. Commonly paramorphic after the cubic high-temperature phase (“argentite”), of original cubic or octahedral habit, to 8 cm. Twinning: Polysynthetic on {111}, may be very complex due to inversion; contact on {101}. Physical Properties: Cleavage: Indistinct. Fracture: Uneven. Tenacity: Sectile. Hardness = 2.0–2.5 VHN = 21–25 (50 g load). D(meas.) = 7.20–7.22 D(calc.) = 7.24 Photosensitive. Optical Properties: Opaque. Color: Iron-black. Streak: Black. Luster: Metallic. Anisotropism: Weak. R: (400) 32.8, (420) 32.9, (440) 33.0, (460) 33.1, (480) 33.0, (500) 32.7, (520) 32.0, (540) 31.2, (560) 30.5, (580) 29.9, (600) 29.2, (620) 28.7, (640) 28.2, (660) 27.6, (680) 27.0, (700) 26.4 ◦ Cell Data: Space Group: P 21/n. a = 4.229 b = 6.931 c = 7.862 β =99.61 Z=4 X-ray Powder Pattern: Synthetic. 2.606 (100), 2.440 (80), 2.383 (75), 2.836 (70), 2.583 (70), 2.456 (70), 3.080 (60) Chemistry: (1) (2) (3) Ag 86.4 87.2 87.06 Cu 0.1 Se 1.6 S 12.0 12.6 12.94 Total 100.0 99.9 100.00 (1) Guanajuato, Mexico; by electron microprobe. (2) Santa Lucia mine, La Luz, Guanajuato, Mexico; by electron microprobe. (3) Ag2S. Polymorphism & Series: The high-temperature cubic form (“argentite”) inverts to acanthite at about 173 ◦C; below this temperature acanthite is the stable phase and forms directly. -

Ore Bin / Oregon Geology Magazine / Journal
.TATE 0 .. O"EGON DUMTtIC..,. , O~ O.~"' ••I .. CIUJ. INDUS""". P'CNtTL.UIO. ORIOON THE ORE.-BIN VOL. ~ NO. , 12 PORTLAND, OREGON Permiuioa is ennttd to rc:print informatton C:Ol'ltain~ herdn. AAy crmit eiwn the Orcaon State I}q)artment of Gmlou' and Mineral Indldtric. for c:ompilinl this inf'ormMJon will be appreciated. vol. t, no . 12 THE ORI.-BIN D..... b.r 1942 Portland, Oregon STATE DEPARTIIENT OF GEOLOGY A IIlIIERAL INDUSTRIES Head Ofti •• : 702 'oodlark Bldg., Portland, Or.go.n State Governing Board Earl K. Nixon Director •• H. Strayer, Ohair.an, Baker Y. If. Libboy Kinins £ns1near Albert Burch lIodford John Eliot Allen Goologist E. B. II&.Naughton Portland H. C. Harrison Spe.tro •• op1st State A8say Laborator1e. 400 i. I Street, Grant. Pass 2102 Court Street, Baker Ray C. fr,.sher Field Geologist Noroan S. 'iagner Field Geologist Robart G. Bassett Assayer Hugh K. Lancaster AS80.yer COCKEYEDEAS Our pre.ise may b. sImply stated: ••••••• 11, never mind t he premise. That can come later. Let'e get on with the dlecu8slon. Con8Ider the followlng: We have been keeping records, 1n WashIngton and elsewhere, for the last 150 years, on almost every conceivable th1n, froa the price of dried apples to the trend in halitosls among rata. From records, graphs are made, • or Can be, If anyone is sufficiently curious. Graphs are wonderful. One oan prove almo.t anything by them. After one haa proved .omething, the chances are good that aomeone else may come along and, using another set of oft1cial records, draw a graph tha.t make. -

Native Silver and Its New Structural Modifications
Native silver and its new structural modifications M.I. Novgorodovo, A.I. Gorshkov, and A.V. Mokhov Native silver is distributed in ores of de- an antimonian variety of native silver, crystal- posits of various genetic types, particularly lizing in the hexagonal system - allargentum gold-silver deposits. Examples of the latter of Ramdohr ( 1962). The structure of allar- are known in Central Asia and in Northeast gentum, containing 8-15% Sb and observed USSR, within the Carpathian-Balkan region, together with dyscrasite as a product of the in the western part of the American Cordillera, decomposition of a solid solution of silver and and in Japan. Judging from the available geo- antimony, can be determined, according to logical information, gold-silver deposits belong Ramdohr, as a solid solution of silver in hexa- to shallow formations and are closely associated gonal closest packing with statistical distribution with products of volcanic activity, located in of antimony, Allargentum is generally accepted volcanic rocks of similar age of the andesite- as a separate phase of the system AgSb. This dacite series, often within the vent facies of is not certain, however, and it is possible to paleovolcanic structures of central type, or in assume that antimony is a stabilizer of the hexa- direct proximity to subvolcanic dikes, conclud- gonal structure characteristic of native silver. ing the volcanic cycle of development of the It thus becomes evident that careful studies region. Ore deposits of this type differ in are required of the structure of native silver, complex mineralogical composition, lengthy starting with the assumption that it can deviate single-stage process of formation. -

54 SKUTTERUDITE FROX{ COBALT. ONTARIOI on Some Specimens
54 THE AMENICAN M]NENALOGIST SKUTTERUDITE FROX{ COBALT. ONTARIOI T. I/. WALKER Uruiuersily oJ Torontn On somespecimens recently obtained from the Temiskaming mine, Cobalt, Ontario, small brilliant crystals resembling smal- tite were observed,imbedded in fragments of soft chloritic or micaceouscountry rock includedin the vein. As is well known, smaltite and chloanthite closelyresemble one another and are commonlyintergrown in the samecrystal. It was with a view to determiningthe relative purity of the supposedsmaltite that this examinationwas undertaken. The principal ore in the vein is smaltite,usually massive, but occasionallyforming rough crystalsup to b or 6 millimeters in diameter. The rest of the vein matter is either calcite or lrag- ments of country rock. The small crystals under investigation seemto representthe latest mineral to form;they are tirr white, and verSzlustrous. The specific gravity, determined on 0.62 gram of carefully selectedcrystals, is 6.29,which is closerto the value usually given for skutterudite (CoAss) than to that for smaltite (CoAsz). The crystals, which seldom exceed a millimeter in diameter, were measured on a Goldschmidt goniometer. The fol- lowing forms were obseryed on each crystal: a(IOO), o(111), d(110), and n(211). The relative development of the forms is shown in figure 1. Nothing observed suggests that the crystals are Frc.1. hemihedral-the faces of the last two formswere not alwayspresent in full, but the omissionsappeared to be quite irregular. These four forms have all been observed on both smaltite and skutterudite. An analysis made on the powdered mineral is shown in I. l Presented at the firrt annual meeting of the Mineralogical Society of America, December 28, 1920. -

Members of the Freibergite-Argentotennantite Series and Associated Minerals from Silvermines, County Tipperary, Ireland
Members of the freibergite-argentotennantite series and associated minerals from Silvermines, County Tipperary, Ireland MAREK A. ZAKRZEWSKI Institute of Earth Sciences, Free University, De Boelelaan 1085, 1081 HV Amsterdam, The Netherlands Abstract Ore microscope and electron microprobe investigations on Zn-Pb-Ag ores from B Zone in Silver mines, Ireland, have shown the presence of two As-Sb-Ag phases; a tetrahedrite-group mineral with composition Cu63Ag3.7Fe0.9Znl.2As2.2Sb2.1S12.7 and proustite-pyrargyrite (Ag3As0.5Sb0.5S3). It is postulated that both phases inherited their As and Sb from primary geocronite (Pb14.5As3.4Sb2.6S23) and that they are metastable. An enrichment in Ni in the ores is due to very fine to submicroscopic intergrowths of gersdorffite (Ni0.6Fe0.4AsS) with geocronite and with galena. KEYWORDS: freibergite, argentotennantite, tetrahedrite group, Silvermines, Ireland. Introduction 'ruby silver', Galena occurs in aggregates up to several mm in diameter in the sphalerite. Tetra THE purpose of the investigations reported here hedrite, geocronite and gersdorffite were recog is a mineralogical explanation of high Ni and Ag nized microscopically. contents in some Pb-Zn ores of the Silvermines The second sample (B4611 B) represents mine, County Tipperary, Ireland. This problem minerals crystallized in brecciated grey dolomite was brought to my attention during a short visit rock. The minerals recognized macroscopically to Ireland in May 1979 at the Geological Depart are (in paragenetical sequence): galena, white ment at Silvermines, Mogul of Ireland Ltd, which dolomite, 'honeyblende' sphalerite, and tetrahed also provided typical specimens from room 4611 rite as crystals up to lOmm in size. It is obviously in B Zone.