FAO Fisheries & Aquaculture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Striped Bass Morone Saxatilis
COSEWIC Assessment and Status Report on the Striped Bass Morone saxatilis in Canada Southern Gulf of St. Lawrence Population St. Lawrence Estuary Population Bay of Fundy Population SOUTHERN GULF OF ST. LAWRENCE POPULATION - THREATENED ST. LAWRENCE ESTUARY POPULATION - EXTIRPATED BAY OF FUNDY POPULATION - THREATENED 2004 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2004. COSEWIC assessment and status report on the Striped Bass Morone saxatilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 43 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Production note: COSEWIC would like to acknowledge Jean Robitaille for writing the status report on the Striped Bass Morone saxatilis prepared under contract with Environment Canada, overseen and edited by Claude Renaud the COSEWIC Freshwater Fish Species Specialist Subcommittee Co-chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Ếgalement disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la situation de bar rayé (Morone saxatilis) au Canada. Cover illustration: Striped Bass — Drawing from Scott and Crossman, 1973. Her Majesty the Queen in Right of Canada 2004 Catalogue No. CW69-14/421-2005E-PDF ISBN 0-662-39840-8 HTML: CW69-14/421-2005E-HTML 0-662-39841-6 Recycled paper COSEWIC Assessment Summary Assessment Summary – November 2004 Common name Striped Bass (Southern Gulf of St. -

Striped Bass
Can you tell the difference between a striped bass, a white bass and a striped bass hybrid? Anglers need to know the differences between these spe- cies because different sizes, seasons and creel limits apply to striped bass and striped bass hybrids, and to white bass. Knowing the differences between these species can also help you better understand Pennsylvania fishes and our wa- ters. These fish belong to the family Moronidae, temperate basses, also known as “true” basses. In Pennsylvania, this family also includes the white perch. Moronidae species are medium-sized to large-sized active predators and prized trophy and sport fishes. Some species live only in fresh water, while others are anadromous they spend much of their lives in salt water or brackish water but return to fresh water to spawn. Striped Bass Morone saxatilis Identification: The striped bass has a smoothly arched pro- file, slimmer and more streamlined than a striped bass hybrid, until it reaches a weight of five to 10 pounds, when its body becomes heavy-looking. The back is olive-green to steely blue- gray, sometimes almost black. The sides are silvery to pale rapidly and stay in brackish bays at the end of their downstream silvery-green, shading to white on the belly. There are seven float. Juveniles spend their first and second summers in the or eight distinct dark stripes that run laterally on the side of tidal Delaware River with most inhabitating that area from the the body. Striped bass have two dorsal fins, the front spiny- Schuylkill River downstream into the state of Delaware. -

Genetic Analysis of Whole Mitochondrial Genome of Lateolabrax Maculatus (Perciformes: Moronidae) Indicates the Presence of Two Populations Along the Chinese Coast
ZOOLOGIA 37: e49046 ISSN 1984-4689 (online) zoologia.pensoft.net RESEARCH ARTICLE Genetic analysis of whole mitochondrial genome of Lateolabrax maculatus (Perciformes: Moronidae) indicates the presence of two populations along the Chinese coast Jie Gong 1,2, Baohua Chen 1,2, Bijun Li 1, Zhixiong Zhou 1, Yue Shi 1, Qiaozhen Ke 1,3, Dianchang Zhang 4, Peng Xu 1,2,3 1Fujian Key Laboratory of Genetics and Breeding of Marine Organisms, Xiamen University. 361000 Xiamen, China. 2Shenzhen Research Institute, Xiamen University. 518000 Shenzhen, China. 3State Key Laboratory of Large Yellow Croaker Breeding, Ningde Fufa Fisheries Company Limited. 352130 Ningde, China. 4South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. 510300 Guangzhou, China. Corresponding author: Peng Xu ([email protected]) http://zoobank.org/B5BBA46C-7FC5-44C3-B9CE-19F8E93FAA1C ABSTRACT. The whole mitochondrial genome of Lateolabrax maculatus (Cuvier, 1828) was used to investigate the reasons for the observed patterns of genetic differentiation among 12 populations in northern and southern China. The haplotype diversity and nucleotide diversity of L. maculatus were 0.998 and 0.00169, respectively. Pairwise FST values between popula- tions ranged from 0.001 to 0.429, correlating positively with geographic distance. Genetic structure analysis and haplotype network analysis indicated that these populations were split into two groups, in agreement with geographic segregation and environment. Tajima’s D values, Fu’s Fs tests and Bayesian skyline plot (BSP) indicated that a demographic expansion event may have occurred in the history of L. maculatus. Through selection pressure analysis, we found evidence of significant negative selection at the ATP6, ND3, Cytb, COX3, COX2 and COX1 genes. -

First Record of F2 Hybrid Striped Bass (Morone Chrysops ♀ × Morone Saxatilis ♂ × Morone Chrysops ♀ × Morone Saxatilis ♂) in Kemer Dam Lake
Turkish Journal of Zoology Turk J Zool (2014) 38: 637-641 http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Research Article doi:10.3906/zoo-1304-42 First record of F2 hybrid striped bass (Morone chrysops ♀ × Morone saxatilis ♂ × Morone chrysops ♀ × Morone saxatilis ♂) in Kemer Dam Lake 1, 2 Volkan KIZAK *, Yusuf GÜNER 1 Fisheries Faculty, Tunceli University, Tunceli, Turkey 2 Fisheries Faculty, Ege University, İzmir, Turkey Received: 26.04.2013 Accepted: 19.03.2014 Published Online: 14.07.2014 Printed: 13.08.2014 Abstract: The morphometric and meristic characters of 2 F2 hybrid striped bass (M. chrysops ♀ × M. saxatilis ♂ × M. chrysops ♀ × M. saxatilis ♂) from the family Moronidae are described. Two specimens were caught from Kemer Dam Lake. The body of the F2 hybrid striped bass was elongated, moderately compressed, and scaly. Dorsal surface and sides were silver and black to olive-gray, and the abdomen was white in color. Four or 5 longitudinal broken stripes ran above the lateral line to the caudal fin. The stripes were less visible behind the pectoral fins and below the lateral line. The bodies of the2 F specimens were deeper than 1/4 the fork length. The 2 dorsal fins were separated entirely. The first dorsal fin had 8–9 spines, and the second dorsal fin had a spine and 13–14 soft rays. The caudal fin was slightly forked. The anal fin had 3 spines with 12–14 soft rays. One tooth patch was present on the anterior of the tongue. According to our results, the fish were 2F hybrid striped bass offspring of 1F hybrid striped bass, which can reproduce naturally in Turkey. -

Highly Diversified Late Cretaceous Fish Assemblage Revealed by Otoliths (Ripley Formation and Owl Creek Formation, Northeast Mississippi, Usa)
Rivista Italiana di Paleontologia e Stratigrafia (Research in Paleontology and Stratigraphy) vol. 126(1): 111-155. March 2020 HIGHLY DIVERSIFIED LATE CRETACEOUS FISH ASSEMBLAGE REVEALED BY OTOLITHS (RIPLEY FORMATION AND OWL CREEK FORMATION, NORTHEAST MISSISSIPPI, USA) GARY L. STRINGER1, WERNER SCHWARZHANS*2 , GEORGE PHILLIPS3 & ROGER LAMBERT4 1Museum of Natural History, University of Louisiana at Monroe, Monroe, Louisiana 71209, USA. E-mail: [email protected] 2Natural History Museum of Denmark, Zoological Museum, Universitetsparken 15, DK-2100, Copenhagen, Denmark. E-mail: [email protected] 3Mississippi Museum of Natural Science, 2148 Riverside Drive, Jackson, Mississippi 39202, USA. E-mail: [email protected] 4North Mississippi Gem and Mineral Society, 1817 CR 700, Corinth, Mississippi, 38834, USA. E-mail: [email protected] *Corresponding author To cite this article: Stringer G.L., Schwarzhans W., Phillips G. & Lambert R. (2020) - Highly diversified Late Cretaceous fish assemblage revealed by otoliths (Ripley Formation and Owl Creek Formation, Northeast Mississippi, USA). Riv. It. Paleontol. Strat., 126(1): 111-155. Keywords: Beryciformes; Holocentriformes; Aulopiformes; otolith; evolutionary implications; paleoecology. Abstract. Bulk sampling and extensive, systematic surface collecting of the Coon Creek Member of the Ripley Formation (early Maastrichtian) at the Blue Springs locality and primarily bulk sampling of the Owl Creek Formation (late Maastrichtian) at the Owl Creek type locality, both in northeast Mississippi, USA, have produced the largest and most highly diversified actinopterygian otolith (ear stone) assemblage described from the Mesozoic of North America. The 3,802 otoliths represent 30 taxa of bony fishes representing at least 22 families. In addition, there were two different morphological types of lapilli, which were not identifiable to species level. -

Exploring the Link Between Otolith Growth and Function Along the Biological Continuum in the Context of Ocean Acidification Eric D
University of Massachusetts Boston ScholarWorks at UMass Boston Graduate Doctoral Dissertations Doctoral Dissertations and Masters Theses 6-1-2014 Exploring the Link between Otolith Growth and Function along the Biological Continuum in the Context of Ocean Acidification Eric D. Wilcox Freeburg University of Massachusetts Boston Follow this and additional works at: http://scholarworks.umb.edu/doctoral_dissertations Part of the Environmental Sciences Commons, Geology Commons, Mineral Physics Commons, and the Oceanography Commons Recommended Citation Wilcox Freeburg, Eric D., "Exploring the Link between Otolith Growth and Function along the Biological Continuum in the Context of Ocean Acidification" (2014). Graduate Doctoral Dissertations. Paper 164. This Open Access Dissertation is brought to you for free and open access by the Doctoral Dissertations and Masters Theses at ScholarWorks at UMass Boston. It has been accepted for inclusion in Graduate Doctoral Dissertations by an authorized administrator of ScholarWorks at UMass Boston. For more information, please contact [email protected]. EXPLORING THE LINK BETWEEN OTOLITH GROWTH AND FUNCTION ALONG THE BIOLOGICAL CONTINUUM IN THE CONTEXT OF OCEAN ACIDIFICATION A Dissertation Presented by ERIC D. WILCOX FREEBURG Submitted to the Office of Graduate Studies, University of Massachusetts Boston in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY June 2014 Environmental Sciences Program © 2014 by Eric D. Wilcox Freeburg All rights reserved EXPLORING THE LINK BETWEEN OTOLITH GROWTH AND FUNCTION ALONG THE BIOLOGICAL CONTINUUM IN THE CONTEXT OF OCEAN ACIDIFICATION A Dissertation Presented by ERIC D. WILCOX FREEBURG Approved as to style and content by: ________________________________________________ Robyn Hannigan, Dean Chairperson of Committee ________________________________________________ William E. -

Striped Bass,Morone Saxatilis
COSEWIC Assessment and Status Report on the Striped Bass Morone saxatilis Southern Gulf of St. Lawrence population Bay of Fundy population St. Lawrence River population in Canada Southern Gulf of St. Lawrence population - SPECIAL CONCERN Bay of Fundy population - ENDANGERED St. Lawrence River population – ENDANGERED 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Striped Bass Morone saxatilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. iv + 82 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 2004. COSEWIC assessment and status report on the Striped Bass Morone saxatilis inCanada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 43 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge Jean-François Bourque and Valerie Tremblay for writing the status report on the Striped Bass, Morone saxatilis, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Dr. Eric Taylor, Co-chair of the COSEWIC Freshwater Fishes Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Bar rayé (Morone saxatilis) au Canada. Cover illustration/photo: Striped Bass — Illustration from Scott and Crossman, 1973. -

Contributions to the Life History of the Yellow Bass, Roccus Mississippiensis (Jordan and Eigenmann), in Clear Lake, Iowa Gary James Atchison Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1967 Contributions to the life history of the yellow bass, Roccus Mississippiensis (Jordan and Eigenmann), in Clear Lake, Iowa Gary James Atchison Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Aquaculture and Fisheries Commons, and the Behavior and Ethology Commons Recommended Citation Atchison, Gary James, "Contributions to the life history of the yellow bass, Roccus Mississippiensis (Jordan and Eigenmann), in Clear Lake, Iowa" (1967). Retrospective Theses and Dissertations. 16858. https://lib.dr.iastate.edu/rtd/16858 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. CONTRIBUTIONS TO THE LIFE HISTORY OF THE YELLOrl BASS, ROCCUS MISSISSIPPIENSIS (JORDAN AND EIGENNANN). IN CLEAR LAKE, IOWA by Gary James Atchison A Thesis Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of 11ASTER OF SCIENCE Hajor Subject: Fisheries Biology Approved: Signatures have been redacted for privacy ,rsity Of Science and Technology Ames, Iowa 11 TABLE OF CONTENTS Page INTRODUCTION 1 DESCRIPTION OF THE STUDY AREA 6 Clear Lake 6 MATERIALS AND METHODS -
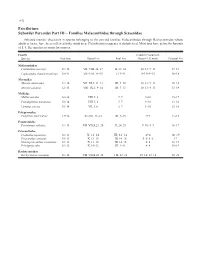
Perciformes Suborder Percoidei Part III – Families Malacanthidae
1152 Perciformes Suborder Percoidei Part III – Families Malacanthidae through Sciaenidae Selected meristic characters in species belonging to the percoid families Malacanthidae through Rachycentridae whose adults or larvae have been collected in the study area. Classification sequence is alphabetical. Most taxa have pelvic fin formula of I, 5. See species accounts for sources. Family Caudal (Procurrent, Species Vertebrae Dorsal Fin Anal Fin Dorsal + Ventral) Pectoral Fin Malacanthidae Caulolatilus microps 11+16 VII–VIII, 24–27 II, 22–24 10–13+9–13 17–18 Lopholatilus chamaeleonticeps 10+14 VII–VIII, 14–15 I, 13–14 9–13+9–13 16–18 Moronidae Morone americana 11+14 VII–XI, I, 11–13 III, 9–10 10–13+9–13 10–18 Morone saxatilis 12+13 VIII–IX, I, 9–14 III, 7–13 10–13+9–13 13–19 Mullidae Mullus auratus 10+14 VIII, I, 8 I, 7 9+10 15–17 Pseudupeneus maculatus 10+14 VIII, I, 8 I, 7 9+10 13–16 Upeneus parvus 10+14 VII, I, 8 I, 7 9+10 15–16 Polyprionidae Polyprion americanus 13+14 XI–XII, 11–12 III, 9–10 9+9 17–18 Pomatomidae Pomatomus saltatrix 11+15 VII–VIII,I,23–28 II, 24–29 9–10+8–9 16–17 Priacanthidae Cookeolus japonicus 10+13 X, 12–14 III, 12–14 4+4 18–19 Priacanthus arenatus 10+13 X, 13–15 III, 14–16 5–6+5–6 17 Heteropriacanthus cruentatus 10+13 X, 13–14 III, 14–15 4+4 18–19 Pristigenys alta 10+13 X, 10–12 III, 9–11 4+4 16–19 Rachycentridae Rachycentron canadum 11+14 VII–VIII,I,26–34 I–II, 22–28 15–16+12–14 20–21 Early Stages of Fishes in the Western North Atlantic Ocean 1153 Perciformes Suborder Percoidei Part III – Families Malacanthidae through Sciaenidae Selected meristic characters in species belonging to the percoid family Sciaenidae whose adults or larvae have been col- lected in the study area. -

Lagniappe Editor Glenn Thomas at Gthomas@ Agctr.Lsu.Edu
Cooperative Extension Service R. Glenn Thomas, Ph.D. Associate Professor Fisheries School of Renewable Natural Resources Room 227 RNR Bldg., LSU Phone: 225.578.0771 Fax: 225. 578.4227 Baton Rouge, LA 70803 [email protected] Web site: www.lsuagcenter.com Research and Extension Programs Agriculture Economic/Community Development Environment/Natural Resources Families/Nutrition/Health 4-H Youth Programs September 1, 2007 Volume 31, No. 9 North Atlantic Cod Collapse Reevaluated The collapse of North Atlantic cod fishery is widely described as the perfect example of overfishing and mismanagement. Few environmental reporters fail to mention this disaster when discussing any ocean fisheries management efforts. Current re-analyses of the causes for the cod collapse focus on “a range of social and political factors that were implicated in the collapse of the stocks, including overfishing, government mismanagement and the disregard of scientific uncertainty.” However, a new comprehensive analysis of the cod populations of the North Atlantic has taken another approach: looking for similarities and differences in the various discrete stocks throughout the North Atlantic, while measuring differences in growth and reproduction in each stock. When commercial fishery effects are added to this analysis a surprising new conclusion becomes apparent: Environmental factors have been an overwhelming influence all along. The study by Brian Rothschild, published in the Transactions of the American Fisheries Society, finds that a strong negative environmental signal, probably associated with plankton dynamics, was a likely suspect in the cod’s disappearance. Spawning stock biomass (SSB - total weight of mature fish) tracked similar trends in all 11 cod stocks: declining from the 1960s through 1975, then increasing through 1985, then falling drastically through the 1990s and remaining low afterwards. -

Full Text in Pdf Format
Vol. 43: 281–289, 2020 ENDANGERED SPECIES RESEARCH Published November 5 https://doi.org/10.3354/esr01074 Endang Species Res OPEN ACCESS Movements of juvenile and sub-adult striped bass Morone saxatilis in the Saint John River, New Brunswick, Canada S. N. Andrews1,*, T. Linnansaari1,2, N. M. Leblanc3, S. A. Pavey3, R. A. Curry1,2 1Canadian Rivers Institute, Department of Biology, 2Faculty of Forestry and Environmental Management, University of New Brunswick, PO Box 4400, Fredericton, New Brunswick E3B 5A3, Canada 3Canadian Rivers Institute, Biological Sciences Department, University of New Brunswick Saint John, 100 Tucker Park Rd., Saint John, New Brunswick E2L 4L5, Canada ABSTRACT: Juvenile striped bass (age-1) of distinct genetic ancestry were re-discovered in the Saint John River, New Brunswick in 2014 after a 35 yr hiatus of recognition. These juveniles were determined to be highly genetically divergent from all possible source populations, hypothesized to be of Saint John River ancestry, and thus considered evidence of the continued existence of the native stock. Successful recruitment of strong year classes of striped bass within the Saint John River, however, appears to be infrequent. We acoustically tagged and tracked juvenile and sub- adult striped bass (n = 37; age 2−4) in the Saint John River in both 2015 and 2016, and identified summer feeding and overwintering habitats that established an in-river residency. Following decades of poor or no recruitment, it is now imperative that managers quickly include monitoring of juvenile and sub-adult striped bass and protection of their habitats in the conservation and recovery efforts for Saint John River striped bass stock. -

Proceedings of the Indiana Academy of Science
76 1891. Jordan. Eelations of temperature to vertebrae among fishes. Proc. U. S. Nat. Mus. 1891, pp. 107-120. 1891. BoUman, Charles Harvey. Review of the Centrarchida', or fresh water sun-fishes of North America. Kept. Comm. Fish and Fisheries 1888. 1884. Kirsch. Bull. U. S. Fish Comm. 18!i4. 1894. Eigenmann. The Fishes of Indiana. Proc. Ind. Acad. Sci. III. 1894. XJlrey, A. B. The Fishes of Wabash County. Proc. Ind. Acad. Sci. Ill, THE FISHES OF INDIANA. * By Carl H. Eigenmann and Charles H. Beeson. We have compiled a list of the species of fishes so far found in Indiana. After the names, scientific and common, we give a list of all the Indiana localities and authority for the locality. The dates with the authority taken with the bibliogi'aphy of Indiana fishes will probably be sufficient to enable anyone to look up any of the citations. In a few cases no date is given with the authority. This indicates that no account has been published of the collections made. I. U. Coll. indicates that specimens from that locality are in the collections of the Indiana University. The localities are arranged in the same order observed in the list of streams which precedes the list of fishes. We give only those streams in which collections have been made. Following the name of the stream we give the name and date of the recorder for the given stream. List of Localities Explored. The Ohio Kiver. White Water River, Brookville, Franklin county—Evermann, 1886. Laughery Creek, Milton, Ohio county—Jenkins, 1888.