Full Text in Pdf Format
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Striped Bass Morone Saxatilis
COSEWIC Assessment and Status Report on the Striped Bass Morone saxatilis in Canada Southern Gulf of St. Lawrence Population St. Lawrence Estuary Population Bay of Fundy Population SOUTHERN GULF OF ST. LAWRENCE POPULATION - THREATENED ST. LAWRENCE ESTUARY POPULATION - EXTIRPATED BAY OF FUNDY POPULATION - THREATENED 2004 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2004. COSEWIC assessment and status report on the Striped Bass Morone saxatilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 43 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Production note: COSEWIC would like to acknowledge Jean Robitaille for writing the status report on the Striped Bass Morone saxatilis prepared under contract with Environment Canada, overseen and edited by Claude Renaud the COSEWIC Freshwater Fish Species Specialist Subcommittee Co-chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Ếgalement disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la situation de bar rayé (Morone saxatilis) au Canada. Cover illustration: Striped Bass — Drawing from Scott and Crossman, 1973. Her Majesty the Queen in Right of Canada 2004 Catalogue No. CW69-14/421-2005E-PDF ISBN 0-662-39840-8 HTML: CW69-14/421-2005E-HTML 0-662-39841-6 Recycled paper COSEWIC Assessment Summary Assessment Summary – November 2004 Common name Striped Bass (Southern Gulf of St. -

Exploring the Link Between Otolith Growth and Function Along the Biological Continuum in the Context of Ocean Acidification Eric D
University of Massachusetts Boston ScholarWorks at UMass Boston Graduate Doctoral Dissertations Doctoral Dissertations and Masters Theses 6-1-2014 Exploring the Link between Otolith Growth and Function along the Biological Continuum in the Context of Ocean Acidification Eric D. Wilcox Freeburg University of Massachusetts Boston Follow this and additional works at: http://scholarworks.umb.edu/doctoral_dissertations Part of the Environmental Sciences Commons, Geology Commons, Mineral Physics Commons, and the Oceanography Commons Recommended Citation Wilcox Freeburg, Eric D., "Exploring the Link between Otolith Growth and Function along the Biological Continuum in the Context of Ocean Acidification" (2014). Graduate Doctoral Dissertations. Paper 164. This Open Access Dissertation is brought to you for free and open access by the Doctoral Dissertations and Masters Theses at ScholarWorks at UMass Boston. It has been accepted for inclusion in Graduate Doctoral Dissertations by an authorized administrator of ScholarWorks at UMass Boston. For more information, please contact [email protected]. EXPLORING THE LINK BETWEEN OTOLITH GROWTH AND FUNCTION ALONG THE BIOLOGICAL CONTINUUM IN THE CONTEXT OF OCEAN ACIDIFICATION A Dissertation Presented by ERIC D. WILCOX FREEBURG Submitted to the Office of Graduate Studies, University of Massachusetts Boston in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY June 2014 Environmental Sciences Program © 2014 by Eric D. Wilcox Freeburg All rights reserved EXPLORING THE LINK BETWEEN OTOLITH GROWTH AND FUNCTION ALONG THE BIOLOGICAL CONTINUUM IN THE CONTEXT OF OCEAN ACIDIFICATION A Dissertation Presented by ERIC D. WILCOX FREEBURG Approved as to style and content by: ________________________________________________ Robyn Hannigan, Dean Chairperson of Committee ________________________________________________ William E. -

Striped Bass,Morone Saxatilis
COSEWIC Assessment and Status Report on the Striped Bass Morone saxatilis Southern Gulf of St. Lawrence population Bay of Fundy population St. Lawrence River population in Canada Southern Gulf of St. Lawrence population - SPECIAL CONCERN Bay of Fundy population - ENDANGERED St. Lawrence River population – ENDANGERED 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Striped Bass Morone saxatilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. iv + 82 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 2004. COSEWIC assessment and status report on the Striped Bass Morone saxatilis inCanada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 43 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge Jean-François Bourque and Valerie Tremblay for writing the status report on the Striped Bass, Morone saxatilis, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Dr. Eric Taylor, Co-chair of the COSEWIC Freshwater Fishes Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Bar rayé (Morone saxatilis) au Canada. Cover illustration/photo: Striped Bass — Illustration from Scott and Crossman, 1973. -

Contributions to the Life History of the Yellow Bass, Roccus Mississippiensis (Jordan and Eigenmann), in Clear Lake, Iowa Gary James Atchison Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1967 Contributions to the life history of the yellow bass, Roccus Mississippiensis (Jordan and Eigenmann), in Clear Lake, Iowa Gary James Atchison Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Aquaculture and Fisheries Commons, and the Behavior and Ethology Commons Recommended Citation Atchison, Gary James, "Contributions to the life history of the yellow bass, Roccus Mississippiensis (Jordan and Eigenmann), in Clear Lake, Iowa" (1967). Retrospective Theses and Dissertations. 16858. https://lib.dr.iastate.edu/rtd/16858 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. CONTRIBUTIONS TO THE LIFE HISTORY OF THE YELLOrl BASS, ROCCUS MISSISSIPPIENSIS (JORDAN AND EIGENNANN). IN CLEAR LAKE, IOWA by Gary James Atchison A Thesis Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of 11ASTER OF SCIENCE Hajor Subject: Fisheries Biology Approved: Signatures have been redacted for privacy ,rsity Of Science and Technology Ames, Iowa 11 TABLE OF CONTENTS Page INTRODUCTION 1 DESCRIPTION OF THE STUDY AREA 6 Clear Lake 6 MATERIALS AND METHODS -
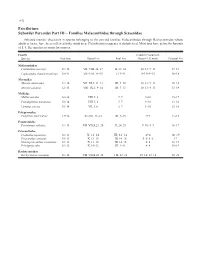
Perciformes Suborder Percoidei Part III – Families Malacanthidae
1152 Perciformes Suborder Percoidei Part III – Families Malacanthidae through Sciaenidae Selected meristic characters in species belonging to the percoid families Malacanthidae through Rachycentridae whose adults or larvae have been collected in the study area. Classification sequence is alphabetical. Most taxa have pelvic fin formula of I, 5. See species accounts for sources. Family Caudal (Procurrent, Species Vertebrae Dorsal Fin Anal Fin Dorsal + Ventral) Pectoral Fin Malacanthidae Caulolatilus microps 11+16 VII–VIII, 24–27 II, 22–24 10–13+9–13 17–18 Lopholatilus chamaeleonticeps 10+14 VII–VIII, 14–15 I, 13–14 9–13+9–13 16–18 Moronidae Morone americana 11+14 VII–XI, I, 11–13 III, 9–10 10–13+9–13 10–18 Morone saxatilis 12+13 VIII–IX, I, 9–14 III, 7–13 10–13+9–13 13–19 Mullidae Mullus auratus 10+14 VIII, I, 8 I, 7 9+10 15–17 Pseudupeneus maculatus 10+14 VIII, I, 8 I, 7 9+10 13–16 Upeneus parvus 10+14 VII, I, 8 I, 7 9+10 15–16 Polyprionidae Polyprion americanus 13+14 XI–XII, 11–12 III, 9–10 9+9 17–18 Pomatomidae Pomatomus saltatrix 11+15 VII–VIII,I,23–28 II, 24–29 9–10+8–9 16–17 Priacanthidae Cookeolus japonicus 10+13 X, 12–14 III, 12–14 4+4 18–19 Priacanthus arenatus 10+13 X, 13–15 III, 14–16 5–6+5–6 17 Heteropriacanthus cruentatus 10+13 X, 13–14 III, 14–15 4+4 18–19 Pristigenys alta 10+13 X, 10–12 III, 9–11 4+4 16–19 Rachycentridae Rachycentron canadum 11+14 VII–VIII,I,26–34 I–II, 22–28 15–16+12–14 20–21 Early Stages of Fishes in the Western North Atlantic Ocean 1153 Perciformes Suborder Percoidei Part III – Families Malacanthidae through Sciaenidae Selected meristic characters in species belonging to the percoid family Sciaenidae whose adults or larvae have been col- lected in the study area. -

Proceedings of the Indiana Academy of Science
76 1891. Jordan. Eelations of temperature to vertebrae among fishes. Proc. U. S. Nat. Mus. 1891, pp. 107-120. 1891. BoUman, Charles Harvey. Review of the Centrarchida', or fresh water sun-fishes of North America. Kept. Comm. Fish and Fisheries 1888. 1884. Kirsch. Bull. U. S. Fish Comm. 18!i4. 1894. Eigenmann. The Fishes of Indiana. Proc. Ind. Acad. Sci. III. 1894. XJlrey, A. B. The Fishes of Wabash County. Proc. Ind. Acad. Sci. Ill, THE FISHES OF INDIANA. * By Carl H. Eigenmann and Charles H. Beeson. We have compiled a list of the species of fishes so far found in Indiana. After the names, scientific and common, we give a list of all the Indiana localities and authority for the locality. The dates with the authority taken with the bibliogi'aphy of Indiana fishes will probably be sufficient to enable anyone to look up any of the citations. In a few cases no date is given with the authority. This indicates that no account has been published of the collections made. I. U. Coll. indicates that specimens from that locality are in the collections of the Indiana University. The localities are arranged in the same order observed in the list of streams which precedes the list of fishes. We give only those streams in which collections have been made. Following the name of the stream we give the name and date of the recorder for the given stream. List of Localities Explored. The Ohio Kiver. White Water River, Brookville, Franklin county—Evermann, 1886. Laughery Creek, Milton, Ohio county—Jenkins, 1888. -

Fish Taphonomy and Environmental Inference in Paleolimnology
Palaeogeography, Palaeoclimatology, Palaeoecology, 62 (1988): 577 592 577 Elsevier Science Publishers B.V., Amsterdam -- Printed in The Netherlands FISH TAPHONOMY AND ENVIRONMENTAL INFERENCE IN PALEOLIMNOLOGY R. L. ELDER and G. R. SMITH Department of Geology, Oberlin College, Oberlin, OH 44074 (U.S.A.) Museum of Paleontology, University of Michigan, Ann Arbor, MI 48109 (U.S.A.) (Accepted for publication April 24, 1986) Abstract Elder, R. L. and Smith, G. R., 1988. Fish taphonomy and environmental inference in paleolimnology. Palaeogeogr., Palaeoclimatol., Palaeoecol., 62: 577-592. The contribution of fish studies to paleoecology generally takes the form of (1) inference from analogies in modern fish faunas and (2) fish taphonomy -- the pattern of death and dispersal of bones. (1) Modern fish faunas and associated organisms provide taxonomic, ecological, or functional analogues for interpretation of ancient limiting factors and behaviors. These inferences presume taxonomic conservatism. They also presume functional relationships between morphological form and feeding mode or habitat. They become weak with increased geologic age or phyletic distance between ancient subject and modern analogue. (2) Fish taphonomy may contribute information about limnology, community composition, life history, mortality, depositional environment, and preservation. Taphonomic reconstruc- tion of ecology and preservation depends on the applicability of analogous processes in modern ecology and limnology. In aquatic taphonomy, temperature is the most important factor in determining the fate of a carcass. Above about 16°C (depending on depth and pressure), most carcasses are made buoyant by bacterial decay gases and are transported to the surface where they may decay further and fall piecemeal into deepwater environments, or drift to beach environments where wave energy disarticulates, abrades, and scatters the bones. -

Current Happenings in Paleontology at The
Inside The Newsletter of Highlights Calvert Marine Museum • News from the CMM • Rhino Foot Bone Found Fossil Club Paleontology Department • Minutes from previous Volume 18 • Number 4 • Past and Present Piscivorous meetings December 2003 Food-chains • Look what Isabel did! Whole Number 61 • Renew Your Club Membership Now The CURRENT HAPPENINGS IN Fascinating Fossil Finds PALEONTOLOGY In late September, Hurricane Isabel wiped the AT THE, CALVERT MARINE cliffs clean! Slumps blocks, no matter how large, MUSEUM were completely removed. Consequently, several dolphin and whale fossils came to light. Scientist's FROM THE CURATOR Cliffs resident, Ruth Ghrist pointed out a partially exposed braincase of a cetothere whale. On ~ September 24th, with help from Bill Counterman, _pcoming club event: Saturday, January 10th, Vanessa Ridgley, Kimberly Connor, Pam and 2004. This second in our series of lectures on Bob Platt, Mike Fulcher and Steve KuIIen, we dinosaurs from Madagascar will feature: successfully removed' a large baleen whale skull from Bed 14 of the Calvert Formation (Figures 1-3). Dr. Catherine A. Forster, (Department of Anatomical Sciences Stony Brook University) will lecture on "The Land That Time Forgot: Madagascar at the end of the Age of Dinosaurs" Her lecture will begin at 2:30 pm in the Museum's auditorium on Saturday, January 10th, 2004. This free public lecture will follow our refular 12:30 club meeting held in the 3r floor lounge in the main exhibits building. Figure 1. From left to right, Kimberley Connor and Pam Platt remove sediment from around the baleen whale skull. (Photo: S. Godfrey) 2 The Ecphora August 2003 New paleontology volunteer, Anna Fuller continues to prepare the skull. -

The Late Eocene Flora of Kučlín Near Bílina in North Bohemia Revisited
SBORNÍK NÁRODNÍHO MUZEA V PRAZE ACTA MUSEI NATIONALIS PRAGAE Řada B – Přírodní vědy • sv. 67 • 2011 • čís. 3–4 • s. 83–144 Series B – Historia Naturalis • vol. 67 • 2011 • no. 3–4 • pp. 83–144 THE LATE EOCENE FLORA OF KUČLÍN NEAR BÍLINA IN NORTH BOHEMIA REVISITED ZLATKO KVAČEK Institute of Geology and Palaeontology, Faculty of Science, Charles University in Prague, Albertov 6, 128 43 Prague 2, Czech Republic; e-mail: [email protected] VASILIS TEODORIDIS Department of Biology and Environmental Education Studies, Faculty of Education, Charles University in Prague, M.D. Ret- tigové 4, 116 39 Prague 1, Czech Republic; e-mail: [email protected] Kvaček, Z., Teodoridis, V. (2011): The Late Eocene flora of Kučlín near Bílina in North Bohemia revisited – Acta. Mus. Nat. Pragae, Ser. B, Hist. Nat., 67(3–4): 83–144, Praha. ISSN 0036-5343. Abstract. A detailed survey of the Late Eocene flora of the diatomite of Kučlín, the Trupelník Hill in North Bohemia, České středohoří Mountains, is given based on the morphological study of most of the so far published macrofossil records since Ettingshausen’s pioneer study and many newly acquired taxa. Both extinct and some modern genera represented mostly by leaf morphotypes and less commonly by fruits and seeds have been encountered. They belong to the ferns (Osmundaceae, Thelypteridaceae, Blechnaceae etc.), conifers (Cupressaceae, Doliostrobaceae) and prevailingly to Angiosperms. Representatives of e.g., Nymphaeaceae, Magnoliaceae, Lauraceae, Platanaceae, Ulmaceae, Fagaceae, Juglandaceae, Fabaceae and some more exotic families such as Icacinaceae, Simaroubaceae and Rutaceae are most numerous, while many belong to extinct groups not assignable to any modern family (e.g., Raskya). -

Candidate Species for Florida Aquaculture: Hybrid Striped Bass, Morone Saxatilis X Morone Chrysops1 Cortney L
FA155 Candidate Species for Florida Aquaculture: Hybrid Striped Bass, Morone saxatilis x Morone chrysops1 Cortney L. Ohs, Christian L. Miller, and R. LeRoy Creswell2 General Description The physical appearance of a hybrid striped bass is a combination of the parental species. The body is slightly Hybrid striped bass are a cross between two species, the compressed and commonly shaped more like a white striped bass (Morone saxatilis) and the white bass (Morone bass. In the hybrid, lateral stripes are irregular or broken chrysops) (Figure1). The genus Morone belongs to the behind the pectoral fins and above the lateral line, while a family Percichthyidae of the order Perciformes. The first pure striped bass has unbroken stripes. Hybrids have two cross was formed by stripping eggs from a female striped dorsal fins, a spinous fin with 8–9 spines and a soft-rayed bass and fertilizing them with sperm from a male white fin with one spine and 13–14 rays. The caudal fin is forked bass. This cross, called the “original cross,” was first done in and the lobes are pointed. The anal fin has three spines and South Carolina in 1965 and now is properly referred to as 9–13 soft rays. Generally, palmetto bass larvae are slightly a palmetto bass. In 1973, the cross between a female white larger than sunshine bass larvae and have a wider mouth bass and male striped bass was again created by stripping gape. There are no observable physical differences between eggs and fertilizing them with sperm. This cross, called the the different crosses once a fish reaches a juvenile stage. -

Bulletin of the United States Fish Commission Seattlenwf
%-A REVIEW OF THE GENERA AND SPECIES OF SERRANID& FOUND IN THE WATERS OF AMERICA AND EUROPE. BY DAVID STARR JORDAN AND CARL H. EIGENMANN. The family of h’evranida: includes many of our most important food fishes. The group comprises the various species popularly recognized as salt-water p rch at~d1?a8s, the groupers, garrupas, hinds, cabrillas, jaw-fishes, together with the striped bass of digerent species which inhabit or asoend our rivers. Nearly a hundred species are fouud in North American waters, aud of these every one, according to its size, is valuable as food. Some of them, popularly known as “jew-fishes,” are among the very largest of spiny-rayed fishes, aud many of the smaller forms are remarkable for the brilliancy of their coloration. This present paper contains an enumeration of the genera and species belonging to the family of Serranidct! found in tbs waters of America and Europe, together with the syuonymy of each, and analytical keys by which the different groups may be dis- tinguished. An earlier paper by Professors Jordan and Swain (Proc. U. 5. Nat. Mus., 1884, p. 358-411) has served as the basis of our studies of the Epinephelilza:,but this paper has been supplemented by the study of a very much larger amoirnt of material, aud the whole group of Berranidm has been brought under co nsideration. We have examined all the specimens of Serranidct! now contained in the Museum of Uoinparative Zoology at Uambridge, Massachusetts,* and all that are in the iniiseuin of the Indiana University. A large part of the material in the U. -

Callorhinchus Milii
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1382 Development and three- dimensional histology of vertebrate dermal fin spines ANNA JERVE ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-554-9596-1 UPPSALA urn:nbn:se:uu:diva-286863 2016 Dissertation presented at Uppsala University to be publicly examined in Lindahlsalen, Evolutionary Biology Center, Norbyvägen 18A, Uppsala, Monday, 13 June 2016 at 09:00 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Philippe Janvier (Muséum National d'Histoire Naturelle, Paris, France). Abstract Jerve, A. 2016. Development and three-dimensional histology of vertebrate dermal fin spines. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1382. 53 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9596-1. Jawed vertebrates (gnathostomes) consist of two clades with living representatives, the chondricthyans (cartilaginous fish including sharks, rays, and chimaeras) and the osteichthyans (bony fish and tetrapods), and two fossil groups, the "placoderms" and "acanthodians". These extinct forms were thought to be monophyletic, but are now considered to be paraphyletic partly due to the discovery of early chondrichthyans and osteichthyans with characters that had been previously used to define them. Among these are fin spines, large dermal structures that, when present, sit anterior to both median and/or paired fins in many extant and fossil jawed vertebrates. Making comparisons among early gnathostomes is difficult since the early chondrichthyans and "acanthodians", which have less mineralized skeleton, do not have large dermal bones on their skulls. As a result, fossil fin spines are potential sources for phylogenetic characters that could help in the study of the gnathostome evolutionary history.