Targeting Intracellular P-Aminobenzoic Acid Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Para-Aminosalicylic Acid – Biopharmaceutical, Pharmacological
Para-aminosalicylic acid – biopharmaceutical, pharmacological... PHARMACIA, vol. 62, No. 1/2015 25 PARA-AMINOSALICYLIC ACID – BIOPHARMACEUTICAL, PHARMACO- LOGICAL, AND CLINICAL FEATURES AND RESURGENCE AS AN ANTI- TUBERCULOUS AGENT G. Momekov*, D. Momekova, G. Stavrakov, Y. Voynikov, P. Peikov Faculty of Pharmacy, Medical University of Sofia, 2 Dunav Str., 1000 Sofia, Bulgaria Summary: Para-aminosalicylic acid (INN aminosalicylic acid; PAS) is a bacteriostatic chemo- therapeutic agent used in the therapy of all forms of tuberculosis, both pulmonary and extrapul- monary, caused by sensitive strains of the mycobacteria resistant to other antituberculotics or if the patient is intolerant towards other drugs. Since its clinical introduction in the late 1940s aminosalicylic acid (PAS) has been a mainstay in the treatment of TB into the 1960s. Along with isoniazid and streptomycin, it was a ‘first-line’ agent for tuberculosis. However, it was plagued by poor gastro-intestinal tolerance and rare but severe allergic reactions. Ethambutol was later shown to be approximately equivalent to PAS in potency, and generally better tolerated than PAS when ethambutol was used at dosages of 25 mg/kg/day or less. Therefore, PAS was replaced by etham- butol as a primary TB drug. However, because of the relative lack of use of PAS over the past 3 decades, most isolates of TB remain susceptible to it. Thus, PAS has experienced a renaissance in the management of patients with multi-drug resistant tuberculosis. Key words: Aminosalicylic acid, Antituberculous agents, MDR-TB, XDR-TB Introduction of treatment the cure rate improved up to 90%. The In 1943 the Swedish chemist Jörgen Lehmann combination of both drugs reduced the selection of (1898-1989) addressed a letter to the managers of resistant strains tremendously [3]. -

Treatment of Drug-Resistant Tuberculosis an Official ATS/CDC/ERS/IDSA Clinical Practice Guideline Payam Nahid, Sundari R
AMERICAN THORACIC SOCIETY DOCUMENTS Treatment of Drug-Resistant Tuberculosis An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline Payam Nahid, Sundari R. Mase, Giovanni Battista Migliori, Giovanni Sotgiu, Graham H. Bothamley, Jan L. Brozek, Adithya Cattamanchi, J. Peter Cegielski, Lisa Chen, Charles L. Daley, Tracy L. Dalton, Raquel Duarte, Federica Fregonese, C. Robert Horsburgh, Jr., Faiz Ahmad Khan, Fayez Kheir, Zhiyi Lan, Alfred Lardizabal, Michael Lauzardo, Joan M. Mangan, Suzanne M. Marks, Lindsay McKenna, Dick Menzies, Carole D. Mitnick, Diana M. Nilsen, Farah Parvez, Charles A. Peloquin, Ann Raftery, H. Simon Schaaf, Neha S. Shah, Jeffrey R. Starke, John W. Wilson, Jonathan M. Wortham, Terence Chorba, and Barbara Seaworth; on behalf of the American Thoracic Society, U.S. Centers for Disease Control and Prevention, European Respiratory Society, and Infectious Diseases Society of America THIS OFFICIAL CLINICAL PRACTICE GUIDELINE WAS APPROVED BY THE AMERICAN THORACIC SOCIETY, THE EUROPEAN RESPIRATORY SOCIETY, AND THE INFECTIOUS DISEASES SOCIETY OF AMERICA SEPTEMBER 2019, AND WAS CLEARED BY THE U.S. CENTERS FOR DISEASE CONTROL AND PREVENTION SEPTEMBER 2019 Background: The American Thoracic Society, U.S. Centers for was judged to be very low, because the data came Disease Control and Prevention, European Respiratory Society, and from observational studies with significant loss to follow-up Infectious Diseases Society of America jointly sponsored this new and imbalance in background regimens between comparator practice guideline on the treatment of drug-resistant tuberculosis groups. Good practices in the management of MDR-TB are (DR-TB). The document includes recommendations on the described. On the basis of the evidence review, a clinical strategy treatment of multidrug-resistant TB (MDR-TB) as well as tool for building a treatment regimen for MDR-TB is also isoniazid-resistant but rifampin-susceptible TB. -

Efficacy and Tolerability of Ethionamide Versus Prothionamide: a Systematic Review
ERJ Express. Published on June 10, 2016 as doi: 10.1183/13993003.00438-2016 RESEARCH LETTER | IN PRESS | CORRECTED PROOF Efficacy and tolerability of ethionamide versus prothionamide: a systematic review To the Editor: To treat multidrug-resistant tuberculosis (MDR-TB), the World Health Organization recommends to include, during the intensive phase of treatment, at least a parenteral agent, a later-generation fluoroquinolone, ethionamide (Eth) (or prothionamide (Pth)), cycloserine (Cs) or p-aminosalicylic acid (PAS) if Cs cannot be used, and pyrazinamide (Pzd) (which is not considered among the aforementioned four probably effective drugs) [1, 2]. In particular, among the four drugs likely to be effective, at least two essential or “core” drugs (one with a good bactericidal and one with a good sterilising activity) and two other “companion” drugs should be administered [3, 4]. In most of the countries where drug susceptibility testing cannot be performed to guide treatment regimen design, standardised second-line treatment regimens are prescribed, based on kanamycin (Km), levofloxacin (Lfx), Eth, Cs and Pzd. Although the regimen is built following the international recommendations, the outcomes remain poor globally [5, 6]. Less than 50–70% of the cases, in fact, achieve treatment success [5, 6], resulting in insufficient control of MDR-TB. It is widely recognised that one frequent cause of poor outcome is treatment default, which is mostly due to the low tolerability of the antituberculosis drugs employed [7]. One of the less tolerated antibiotics is Eth, because of the serious and frequent gastric adverse events [8 9] or of hypothyroidism, which is frequently subclinical. Eth and Pth are thionamide drugs, characterised by a structure similar to isoniazid (Inh). -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2019 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Alphabetical Listing of ATC Drugs & Codes
Alphabetical Listing of ATC drugs & codes. Introduction This file is an alphabetical listing of ATC codes as supplied to us in November 1999. It is supplied free as a service to those who care about good medicine use by mSupply support. To get an overview of the ATC system, use the “ATC categories.pdf” document also alvailable from www.msupply.org.nz Thanks to the WHO collaborating centre for Drug Statistics & Methodology, Norway, for supplying the raw data. I have intentionally supplied these files as PDFs so that they are not quite so easily manipulated and redistributed. I am told there is no copyright on the files, but it still seems polite to ask before using other people’s work, so please contact <[email protected]> for permission before asking us for text files. mSupply support also distributes mSupply software for inventory control, which has an inbuilt system for reporting on medicine usage using the ATC system You can download a full working version from www.msupply.org.nz Craig Drown, mSupply Support <[email protected]> April 2000 A (2-benzhydryloxyethyl)diethyl-methylammonium iodide A03AB16 0.3 g O 2-(4-chlorphenoxy)-ethanol D01AE06 4-dimethylaminophenol V03AB27 Abciximab B01AC13 25 mg P Absorbable gelatin sponge B02BC01 Acadesine C01EB13 Acamprosate V03AA03 2 g O Acarbose A10BF01 0.3 g O Acebutolol C07AB04 0.4 g O,P Acebutolol and thiazides C07BB04 Aceclidine S01EB08 Aceclidine, combinations S01EB58 Aceclofenac M01AB16 0.2 g O Acefylline piperazine R03DA09 Acemetacin M01AB11 Acenocoumarol B01AA07 5 mg O Acepromazine N05AA04 -

Frequently Asked Questions on The
Frequently asked questions on the WHO treatment guidelines for isoniazid-resistant tuberculosis Version: 24 April 2018 The advice in this document has been prepared by the Global TB Programme of the World Health Organization (WHO) and is to be read alongside the WHO treatment guidelines for isoniazid-resistant tuberculosis and its online annexes. See Further reading at end for additional resources. Who are the new WHO treatment guidelines for isoniazid-resistant tuberculosis intended for ? The WHO treatment guidelines for isoniazid-resistant tuberculosis are targeted at health care professionals (doctors, nurses) and other staff involved in TB care in both low and high resource settings. The possibility of drug-resistant disease now needs to be entertained whenever treating TB patients. These guidelines provide technical detail which is helpful in making the best-informed decision in clinical practice and programme management. The answers to the frequently asked questions (FAQ) in this document intend to help implementers with common, practical issues that arise when adopting the new guidance. This document complements the WHO guidelines by elaborating further on certain aspects of implementation that were beyond the scope of the guideline itself. What are the main recommendations of these new guidelines ? The new guidelines recommend that patients with confirmed isoniazid-resistant and rifampicin susceptible tuberculosis (abbreviated to Hr-TB) are treated for 6 months with a regimen composed of rifampicin (R), ethambutol (E), pyrazinamide (Z) and levofloxacin (Lfx). The exclusion of rifampicin resistance ahead of start of this regimen is critical. It is further recommended not to add streptomycin (S) or other injectable agents to the treatment regimen. -

Mycobacterium Tuberculosis Complex Drug Susceptibility Testing Program
Centers for Disease Control and Prevention Model Performance Evaluation Program Mycobacterium tuberculosis Complex Drug Susceptibility Testing Program Report of Results November 2013 Performance Evaluation Survey Mycobacterium tuberculosis Complex Drug Susceptibility Testing Report for November 2013 Survey Purpose The purpose of this report is to present the results of the U.S. Centers for Disease Control and Prevention (CDC) Model Performance Evaluation Program (MPEP) for Mycobacterium tuberculosis complex drug susceptibility testing survey sent to participants in November 2013. Report Content The material in this report was developed and prepared by Cortney Stafford, MPH, MT (ASCP), Health Scientist, Laboratory Capacity Team, NCHHSTP, DTBE, LB Beverly Metchock, DrPH, D(ABMM), Team Lead, Reference Laboratory, NCHHSTP, DTBE, LB Acknowledged contributors: Lois Diem NCHHSTP, DTBE, LB; Mitchell Yakrus NCHHSTP, DTBE, LB; Angela Starks NCHHSTP, DTBE, LB Contact Information Comments and inquiries regarding this report should be directed to [email protected] 404-639-4013 The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. 2 CDC MPEP MTBC DST Report for November 2013 Survey Table of Contents Mycobacterium tuberculosis Complex Drug Susceptibility Testing MPEP Report for November 2013 Survey -

Tuberculosis Drug Information Guide, 2Nd Edition
Tuberculosis Drug Information Guide 2ND EDITION Edmund G. Brown Jr, Governor STATE OF CALIFORNIA Diana S. Dooley, Secretary CALIFORNIA HEALTH & HUMAN SERVICES AGENCY Ron Chapman, MD, MPH, Director DEPARTMENT OF PUBLIC HEALTH Tuberculosis Drug Information Guide 2ND EDITION Edmund G. Brown Jr, Governor STATE OF CALIFORNIA Diana S. Dooley, Secretary CALIFORNIA HEALTH & HUMAN SERVICES AGENCY Ron Chapman, MD, MPH, Director DEPARTMENT OF PUBLIC HEALTH DrugInfo_2ndEd_v9.indd 3 12/5/12 3:55 PM Tuberculosis Drug Information Guide, 2nd edition was created through a collaboration of the Curry International Tuberculosis Center (CITC) and the State of California Department of Public Health, Tuberculosis Control Branch (CDPH). CITC is a project of the University of California, San Francisco, funded by the Centers for Disease Control and Prevention (CDC). The development of this Guide was funded through CDC Cooperative Agreement U52 CCU 900454. Permission is granted for nonprofit educational use and library duplication and distribution. Suggested citation: Curry International Tuberculosis Center and California Department of Public Health, 2012: Tuberculosis Drug Information Guide, 2nd edition. This publication is available on the CITC website: http://www.currytbcenter.ucsf.edu/ tbdruginfo/ Design: Edi Berton Design DrugInfo_2ndEd_v9.indd 4 12/5/12 3:55 PM Contributors Acknowledgments Ann M. Loeffler, MD John S. Bradley, MD Pediatric Infectious Diseases Attending and Director of Pediatric Rady Children’s Hospital San Diego, San Diego, California Hospitalists at Randall Children’s Hospital at Legacy Emanuel, Daniel Deck, Pharm.D. Portland, Oregon Department of Pharmaceutical Services, Pediatric Tuberculosis Consultant, San Francisco General Hospital, California Curry International Tuberculosis Center, San Francisco, California David Forinash, RPh Randall Children’s Hospital at Legacy Emanuel, Portland, Oregon Charles A. -

Antimicrobial Resistance in Bacteria
Cent. Eur. J. Med. • 4(2) • 2009 • 141-155 DOI: 10.2478/s11536-008-0088-9 Central European Journal of Medicine Antimicrobial resistance in bacteria Review Article Katrijn Bockstael*, Arthur Van Aerschot** Laboratory for Medicinal Chemistry, Rega Institute for Medical Research, Katholieke Universiteit Leuven, 3000 Leuven, Belgium Received 9 July 2008; Accepted 17 November 2008 Abstract: The development of antimicrobial resistance by bacteria is inevitable and is considered as a major problem in the treatment of bacterial infections in the hospital and in the community. Despite efforts to develop new therapeutics that interact with new targets, resistance has been reported even to these agents. In this review, an overview is given of the many therapeutic possibilities that exist for treatment of bacterial infections and how bacteria become resistant to these therapeutics. Keywords: Antimicrobial agents • Resistance development • Efflux • Alteration of drug target • Antibacterials © Versita Warsaw and Springer-Verlag Berlin Heidelberg. 1. Introduction 2. Different mechanisms of resistance to antimicrobials The history of humankind can be regarded from a medical point of view as a struggle against infectious 2.1. Intrinsic resistance diseases. Infections were the leading cause of death Bacteria may be inherently resistant to an antimicrobial. th worldwide at the beginning of the 20 century. Since This passive resistance is a consequence of general the discovery of penicillin by Alexander Fleming in adaptive processes that are not necessary linked to a 1929 and the first introduction of the sulpha drugs by given class of antimicrobials. An example of natural Domagk in 1932, the number of new antimicrobials resistance is Pseudomonas aeruginosa, whose low available has increased tremendously between 1940 membrane permeability is likely to be a main reason for and 1960. -

Surveillance for Falsified and Substandard Medicines in Africa and Asia by Local Organizations Using the Low-Cost GPHF Minilab
RESEARCH ARTICLE Surveillance for falsified and substandard medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab Albert Petersen1*, Nadja Held2, Lutz Heide2*, on behalf of the DifaÈm-EPN Minilab Survey Group¶ a1111111111 1 DifaÈm - German Institute for Medical Mission, TuÈbingen, Germany, 2 Pharmaceutical Institute, Eberhard Karls-University TuÈbingen, TuÈbingen, Germany a1111111111 a1111111111 ¶ Membership of the DifaÈm-EPN Minilab Survey Group is provided in the Acknowledgments a1111111111 * [email protected] (AP); [email protected] (LH) a1111111111 Abstract OPEN ACCESS Background Citation: Petersen A, Held N, Heide L, on behalf of Substandard and falsified medical products present a serious threat to public health, espe- the DifaÈm-EPN Minilab Survey Group (2017) cially in low- and middle-income countries. Their identification using pharmacopeial analysis Surveillance for falsified and substandard is expensive and requires sophisticated equipment and highly trained personnel. Simple, medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab. PLoS ONE 12(9): low-cost technologies are required in addition to full pharmacopeial analysis in order to e0184165. https://doi.org/10.1371/journal. accomplish widespread routine surveillance for poor-quality medicines in low- and middle- pone.0184165 income countries. Editor: Yoel Lubell, Mahidol-Oxford Tropical Medicine Research Unit, THAILAND Methods Received: May 30, 2017 Ten faith-based drug supply organizations in seven countries of Africa and Asia were each Accepted: August 19, 2017 equipped with a Minilab of the Global Pharma Health Fund (GPHF, Frankfurt, Germany), suitable for the analysis of about 85 different essential medicines by thin-layer chromatogra- Published: September 6, 2017 phy. -
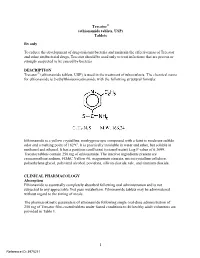
Trecator (Ethionamide Tablets, USP)
Trecator® (ethionamide tablets, USP) Tablets Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of Trecator and other antibacterial drugs, Trecator should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION Trecator® (ethionamide tablets, USP) is used in the treatment of tuberculosis. The chemical name for ethionamide is 2-ethylthioisonicotinamide with the following structural formula: Ethionamide is a yellow crystalline, nonhygroscopic compound with a faint to moderate sulfide odor and a melting point of 162°C. It is practically insoluble in water and ether, but soluble in methanol and ethanol. It has a partition coefficient (octanol/water) Log P value of 0.3699. Trecator tablets contain 250 mg of ethionamide. The inactive ingredients present are croscarmellose sodium, FD&C Yellow #6, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, talc, and titanium dioxide. CLINICAL PHARMACOLOGY Absorption Ethionamide is essentially completely absorbed following oral administration and is not subjected to any appreciable first pass metabolism. Ethionamide tablets may be administered without regard to the timing of meals. The pharmacokinetic parameters of ethionamide following single oral-dose administration of 250 mg of Trecator film-coated tablets under fasted conditions to 40 healthy adult volunteers are provided in Table 1. 1 Reference ID: 3975211 Table 1: Mean (SD) Pharmacokinetic Parameters for Ethionamide Following Single-dose Administration of 250 mg Trecator Film-Coated Tablets to Healthy Adult Volunteers Cmax Tmax AUC (μg/mL) (hrs) (μg•hr/mL) Film-Coated Tablet 2.16 1.02 7.67 (0.61) (0.55) (1.69) Trecator tablets have been reformulated from a sugar-coated tablet to a film-coated tablet. -

Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies
Arrhythmogenic potential of drugs FP7-HEALTH-241679 http://www.aritmo-project.org/ Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies V 1.3 Draft Lead beneficiary: EMC Date: 03/01/2010 Nature: Report Dissemination level: D5.2 Report on Common Study Protocol for Observational Database Studies WP5: Conduct of Additional Observational Security: Studies. Author(s): Gianluca Trifiro’ (EMC), Giampiero Version: v1.1– 2/85 Mazzaglia (F-SIMG) Draft TABLE OF CONTENTS DOCUMENT INFOOMATION AND HISTORY ...........................................................................4 DEFINITIONS .................................................... ERRORE. IL SEGNALIBRO NON È DEFINITO. ABBREVIATIONS ......................................................................................................................6 1. BACKGROUND .................................................................................................................7 2. STUDY OBJECTIVES................................ ERRORE. IL SEGNALIBRO NON È DEFINITO. 3. METHODS ..........................................................................................................................8 3.1.STUDY DESIGN ....................................................................................................................8 3.2.DATA SOURCES ..................................................................................................................9 3.2.1. IPCI Database .....................................................................................................9