Somniosus Microcephalus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Greenland Shark (Somniosus Microcephalus)
Greenland shark (Somniosus microcephalus) feeding behavior on static fishing gear, effect of SMART (Selective Magnetic and Repellent-Treated) hook deterrent technology, and factors influencing entanglement in bottom longlines Scott M. Grant1, Rennie Sullivan1 and Kevin J. Hedges2 1 Centre for Sustainable Aquatic Resources, Memorial University of Newfoundland, St. John’s, NL, Canada 2 Central and Arctic Region, Arctic Aquatic Research Division, Fisheries and Oceans Canada, Winnipeg, MB, Canada ABSTRACT The Greenland Shark (Somniosus microcephalus) is the most common bycatch in the Greenland halibut (Reinhardtius hippoglossoides) bottom longline fishery in Cumberland Sound, Canada. Historically, this inshore fishery has been prosecuted through the ice during winter but winter storms and unpredictable landfast ice conditions since the mid-1990s have led to interest in developing a summer fishery during the ice-free season. However, bycatch of Greenland shark was found to increase substantially with 570 sharks captured during an experimental Greenland halibut summer fishery (i.e., mean of 6.3 sharks per 1,000 hooks set) and mortality was reported to be about 50% due in part to fishers killing sharks that were severely entangled in longline gear. This study investigated whether the SMART (Selective Magnetic and Repellent-Treated) hook technology is a practical deterrent to Greenland shark predation and subsequent bycatch on bottom longlines. Greenland Submitted 1 December 2017 shark feeding behavior, feeding kinematics, and variables affecting entanglement/ 22 April 2018 Accepted disentanglement and release are also described. The SMART hook failed to deter Published 17 May 2018 Greenland shark predation, i.e., all sharks were captured on SMART hooks, some Corresponding author Scott M. -

Mark Report Satellite Tags (Mrpats) to Detail Large-Scale Horizontal Movements of Deep Water Species: First Results for the Greenland Shark (Somniosus Microcephalus)
CORE Metadata, citation and similar papers at core.ac.uk Provided by Scholarship at UWindsor University of Windsor Scholarship at UWindsor Biological Sciences Publications Department of Biological Sciences 2018 Mark report satellite tags (mrPATs) to detail large-scale horizontal movements of deep water species: First results for the Greenland shark (Somniosus microcephalus) Nigel E. Hussey Biological Sciences, University of Windsor Jack Orr Aaron T. Fisk University of Windsor Kevin J. Hedges Steven H. Ferguson See next page for additional authors Follow this and additional works at: https://scholar.uwindsor.ca/biologypub Part of the Biology Commons Recommended Citation Hussey, Nigel E.; Orr, Jack; Fisk, Aaron T.; Hedges, Kevin J.; Ferguson, Steven H.; and Barkley, Amanda N., "Mark report satellite tags (mrPATs) to detail large-scale horizontal movements of deep water species: First results for the Greenland shark (Somniosus microcephalus)" (2018). Deep Sea Research Part I: Oceanographic Research Papers. https://scholar.uwindsor.ca/biologypub/1193 This Article is brought to you for free and open access by the Department of Biological Sciences at Scholarship at UWindsor. It has been accepted for inclusion in Biological Sciences Publications by an authorized administrator of Scholarship at UWindsor. For more information, please contact [email protected]. Authors Nigel E. Hussey, Jack Orr, Aaron T. Fisk, Kevin J. Hedges, Steven H. Ferguson, and Amanda N. Barkley This article is available at Scholarship at UWindsor: https://scholar.uwindsor.ca/biologypub/1193 Author’s Accepted Manuscript Mark report satellite tags (mrPATs) to detail large- scale horizontal movements of deep water species: First results for the Greenland shark (Somniosus microcephalus) Nigel E. -

First Record of Swimming Speed of the Pacific Sleeper Shark Somniosus
Journal of the Marine First record of swimming speed of the Pacific Biological Association of the United Kingdom sleeper shark Somniosus pacificus using a baited camera array cambridge.org/mbi Yoshihiro Fujiwara , Yasuyuki Matsumoto, Takumi Sato, Masaru Kawato and Shinji Tsuchida Original Article Research Institute for Global Change (RIGC), Japan Agency for Marine-Earth Science and Technology (JAMSTEC), 2-15 Yokosuka, Kanagawa 237-0061, Japan Cite this article: Fujiwara Y, Matsumoto Y, Sato T, Kawato M, Tsuchida S (2021). First record of swimming speed of the Pacific Abstract sleeper shark Somniosus pacificus using a baited camera array. Journal of the Marine The Pacific sleeper shark Somniosus pacificus is one of the largest predators in deep Suruga Biological Association of the United Kingdom Bay, Japan. A single individual of the sleeper shark (female, ∼300 cm in total length) was 101, 457–464. https://doi.org/10.1017/ observed with two baited camera systems deployed simultaneously on the deep seafloor in S0025315421000321 the bay. The first arrival was recorded 43 min after the deployment of camera #1 on 21 July 2016 at a depth of 609 m. The shark had several remarkable features, including the Received: 26 July 2020 Revised: 14 April 2021 snout tangled in a broken fishing line, two torn anteriormost left-gill septums, and a parasitic Accepted: 14 April 2021 copepod attached to each eye. The same individual appeared at camera #2, which was First published online: 18 May 2021 deployed at a depth of 603 m, ∼37 min after it disappeared from camera #1 view. Finally, the same shark returned to camera #1 ∼31 min after leaving camera #2. -

Greenland Shark in the Barents Sea: Biology, Distribution and Bycatch
ICES CM 2010/E:26 Greenland shark in the Barents Sea: biology, distribution and bycatch by Rusyaev S.M., Sokolov K.M. and Drevetnyak K.V. E-mail:[email protected] Knipovich Polar Research Institute of Marine Fisheries and Oceanography (PINRO) 6 Knipovich Street, Murmansk, 183038 Russia Introduction Russian fishery for the Greenland shark (Somniosus microcephalus) was commenced in the Barents Sea in the late 19th century (Danilevskiy, 1862) and it was developed there in the early 20th century (Zhilinsky, 1923). Due to the diminishing of the demand for the liver fat of sharks that was caused by the commencement of the synthetic vitamin A production as well as since the coastal fishery was replaced by the oceanic one, the target fishery of the Greenland shark had been virtually ceased by 1961. In the absence of shark fishery, the research on distribution and abundance dynamics of a large predator may be important and urgent in the scope of ecosystem approach which is being developed in fishery science. The research on the Pacific Sleeper shark (Somniosus pacificus) showed that the abundance of representatives from that genus could significantly increase for a short time (Courtney, Sigler, 2002) and pose a threat for fisheries of the main commercial species (Orlov, 2003). Materials and methods Since there are no purposeful researches, the information on shark captures is limited and occasional, as a rule. The material for paper was the data on bycatches of Greenland shark by bottom and midwater gear in the Barents Sea obtained by research and fishing vessels: 233 captures from1981 to the present and 964 captures from January 1968 to August 2009, respectively. -

Synopsis of the Parasites of Fishes of Canada
1 ci Bulletin of the Fisheries Research Board of Canada DFO - Library / MPO - Bibliothèque 12039476 Synopsis of the Parasites of Fishes of Canada BULLETIN 199 Ottawa 1979 '.^Y. Government of Canada Gouvernement du Canada * F sher es and Oceans Pëches et Océans Synopsis of thc Parasites orr Fishes of Canade Bulletins are designed to interpret current knowledge in scientific fields per- tinent to Canadian fisheries and aquatic environments. Recent numbers in this series are listed at the back of this Bulletin. The Journal of the Fisheries Research Board of Canada is published in annual volumes of monthly issues and Miscellaneous Special Publications are issued periodically. These series are available from authorized bookstore agents, other bookstores, or you may send your prepaid order to the Canadian Government Publishing Centre, Supply and Services Canada, Hull, Que. K I A 0S9. Make cheques or money orders payable in Canadian funds to the Receiver General for Canada. Editor and Director J. C. STEVENSON, PH.D. of Scientific Information Deputy Editor J. WATSON, PH.D. D. G. Co«, PH.D. Assistant Editors LORRAINE C. SMITH, PH.D. J. CAMP G. J. NEVILLE Production-Documentation MONA SMITH MICKEY LEWIS Department of Fisheries and Oceans Scientific Information and Publications Branch Ottawa, Canada K1A 0E6 BULLETIN 199 Synopsis of the Parasites of Fishes of Canada L. Margolis • J. R. Arthur Department of Fisheries and Oceans Resource Services Branch Pacific Biological Station Nanaimo, B.C. V9R 5K6 DEPARTMENT OF FISHERIES AND OCEANS Ottawa 1979 0Minister of Supply and Services Canada 1979 Available from authorized bookstore agents, other bookstores, or you may send your prepaid order to the Canadian Government Publishing Centre, Supply and Services Canada, Hull, Que. -

Origins of the Greenland Shark (Somniosus Microcephalus): Impacts of Ice‐Olation and Introgression
Received: 10 February 2017 | Revised: 7 June 2017 | Accepted: 21 July 2017 DOI: 10.1002/ece3.3325 ORIGINAL RESEARCH Origins of the Greenland shark (Somniosus microcephalus): Impacts of ice- olation and introgression Ryan P. Walter1,2 | Denis Roy3 | Nigel E. Hussey4 | Björn Stelbrink5 | Kit M. Kovacs6 | Christian Lydersen6 | Bailey C. McMeans2,7 | Jörundur Svavarsson8 | Steven T. Kessel9 | Sebastián Biton Porsmoguer10 | Sharon Wildes11 | Cindy A. Tribuzio11 | Steven E. Campana8 | Stephen D. Petersen12 | R. Dean Grubbs13 | Daniel D. Heath2 | Kevin J. Hedges14 | Aaron T. Fisk2 1Department of Biological Science, California State University, Fullerton, CA, USA 2Great Lakes Institute for Environmental Research, University of Windsor, Windsor, ON, Canada 3Department of Natural Resources and the Environment, Wildlife and Fisheries Conservation Center and Center for Environmental Sciences and Engineering, University of Connecticut, Storrs, CT, USA 4Biological Sciences, University of Windsor, Windsor, ON, Canada 5Justus Liebig University, Giessen, Germany 6Fram Centre, Norwegian Polar Institute, Tromsø, Norway 7Department of Biology, University of Toronto Mississauga, Mississauga, ON, Canada 8Faculty of Life and Environmental Sciences, University of Iceland, Reykjavík, Iceland 9Department of Fisheries and Wildlife, Michigan State University, East Lansing, MI, USA 10Mediterranean Institute of Oceanography (MIO) UM 110, Aix-Marseille University, CNRS/INSU, Toulon University, IRD, Marseille, France 11Auke Bay Laboratories, AFSC/NMFS/NOAA/DOC, Ted Stevens Marine Research Institute, Juneau, AK, USA 12Conservation and Research Department, Assiniboine Park Zoo, Winnipeg, MB, Canada 13Coastal and Marine Laboratory, Florida State University, St. Teresa, FL, USA 14Arctic Aquatic Research Division, Fisheries and Oceans Canada, Winnipeg, MB, Canada Correspondence Ryan P. Walter, Department of Biological Abstract Science, California State University, Fullerton, Herein, we use genetic data from 277 sleeper sharks to perform coalescent- based CA, USA. -

APPENDIX 1 Classified List of Fishes Mentioned in the Text, with Scientific and Common Names
APPENDIX 1 Classified list of fishes mentioned in the text, with scientific and common names. ___________________________________________________________ Scientific names and classification are from Nelson (1994). Families are listed in the same order as in Nelson (1994), with species names following in alphabetical order. The common names of British fishes mostly follow Wheeler (1978). Common names of foreign fishes are taken from Froese & Pauly (2002). Species in square brackets are referred to in the text but are not found in British waters. Fishes restricted to fresh water are shown in bold type. Fishes ranging from fresh water through brackish water to the sea are underlined; this category includes diadromous fishes that regularly migrate between marine and freshwater environments, spawning either in the sea (catadromous fishes) or in fresh water (anadromous fishes). Not indicated are marine or freshwater fishes that occasionally venture into brackish water. Superclass Agnatha (jawless fishes) Class Myxini (hagfishes)1 Order Myxiniformes Family Myxinidae Myxine glutinosa, hagfish Class Cephalaspidomorphi (lampreys)1 Order Petromyzontiformes Family Petromyzontidae [Ichthyomyzon bdellium, Ohio lamprey] Lampetra fluviatilis, lampern, river lamprey Lampetra planeri, brook lamprey [Lampetra tridentata, Pacific lamprey] Lethenteron camtschaticum, Arctic lamprey] [Lethenteron zanandreai, Po brook lamprey] Petromyzon marinus, lamprey Superclass Gnathostomata (fishes with jaws) Grade Chondrichthiomorphi Class Chondrichthyes (cartilaginous -

Greenland Shark (Somniosus Microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift
Greenland Shark (Somniosus microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift Nielsen, Julius; Christiansen, Jørgen Schou; Grønkjær, Peter; Bushnell, Peter; Steffensen, John Fleng; Kiilerich, Helene Overgaard; Præbel, Kim; Hedeholm, Rasmus Published in: Frontiers in Marine Science DOI: 10.3389/fmars.2019.00125 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Nielsen, J., Christiansen, J. S., Grønkjær, P., Bushnell, P., Steffensen, J. F., Kiilerich, H. O., ... Hedeholm, R. (2019). Greenland Shark (Somniosus microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift. Frontiers in Marine Science, 6, 1-11. [125]. https://doi.org/10.3389/fmars.2019.00125 Download date: 09. Apr. 2020 fmars-06-00125 April 2, 2019 Time: 17:28 # 1 ORIGINAL RESEARCH published: 04 April 2019 doi: 10.3389/fmars.2019.00125 Greenland Shark (Somniosus microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift Julius Nielsen1,2,3,4*, Jørgen Schou Christiansen4,5, Peter Grønkjær6, Peter Bushnell7, John Fleng Steffensen1, Helene Overgaard Kiilerich6, Kim Præbel8 and Rasmus Hedeholm2 1 Marine Biological Section, University of Copenhagen, Helsingør, Denmark, 2 Greenland Institute of Natural Resources, Nuuk, Greenland, 3 Den Blå Planet, National Aquarium Denmark, Kastrup, Denmark, 4 Department of Arctic and Marine Biology, UiT The Arctic University of Norway, -

First Record of Swimming Speed of the Pacific Sleeper Shark Somniosus
Journal of the Marine First record of swimming speed of the Pacific Biological Association of the United Kingdom sleeper shark Somniosus pacificus using a baited camera array cambridge.org/mbi Yoshihiro Fujiwara , Yasuyuki Matsumoto, Takumi Sato, Masaru Kawato and Shinji Tsuchida Original Article Research Institute for Global Change (RIGC), Japan Agency for Marine-Earth Science and Technology (JAMSTEC), 2-15 Yokosuka, Kanagawa 237-0061, Japan Cite this article: Fujiwara Y, Matsumoto Y, Sato T, Kawato M, Tsuchida S (2021). First record of swimming speed of the Pacific Abstract sleeper shark Somniosus pacificus using a baited camera array. Journal of the Marine The Pacific sleeper shark Somniosus pacificus is one of the largest predators in deep Suruga Biological Association of the United Kingdom Bay, Japan. A single individual of the sleeper shark (female, ∼300 cm in total length) was 101, 457–464. https://doi.org/10.1017/ observed with two baited camera systems deployed simultaneously on the deep seafloor in S0025315421000321 the bay. The first arrival was recorded 43 min after the deployment of camera #1 on 21 July 2016 at a depth of 609 m. The shark had several remarkable features, including the Received: 26 July 2020 Revised: 14 April 2021 snout tangled in a broken fishing line, two torn anteriormost left-gill septums, and a parasitic Accepted: 14 April 2021 copepod attached to each eye. The same individual appeared at camera #2, which was First published online: 18 May 2021 deployed at a depth of 603 m, ∼37 min after it disappeared from camera #1 view. Finally, the same shark returned to camera #1 ∼31 min after leaving camera #2. -

DEEPFISHMAN Document 5 : Review of Parasites, Pathogens
DEEPFISHMAN Management And Monitoring Of Deep-sea Fisheries And Stocks Project number: 227390 Small or medium scale focused research action Topic: FP7-KBBE-2008-1-4-02 (Deepsea fisheries management) DEEPFISHMAN Document 5 Title: Review of parasites, pathogens and contaminants of deep sea fish with a focus on their role in population health and structure Due date: none Actual submission date: 10 June 2010 Start date of the project: April 1st, 2009 Duration : 36 months Organization Name of lead coordinator: Ifremer Dissemination Level: PU (Public) Date: 10 June 2010 Review of parasites, pathogens and contaminants of deep sea fish with a focus on their role in population health and structure. Matt Longshaw & Stephen Feist Cefas Weymouth Laboratory Barrack Road, The Nothe, Weymouth, Dorset DT4 8UB 1. Introduction This review provides a summary of the parasites, pathogens and contaminant related impacts on deep sea fish normally found at depths greater than about 200m There is a clear focus on worldwide commercial species but has an emphasis on records and reports from the north east Atlantic. In particular, the focus of species following discussion were as follows: deep-water squalid sharks (e.g. Centrophorus squamosus and Centroscymnus coelolepis), black scabbardfish (Aphanopus carbo) (except in ICES area IX – fielded by Portuguese), roundnose grenadier (Coryphaenoides rupestris), orange roughy (Hoplostethus atlanticus), blue ling (Molva dypterygia), torsk (Brosme brosme), greater silver smelt (Argentina silus), Greenland halibut (Reinhardtius hippoglossoides), deep-sea redfish (Sebastes mentella), alfonsino (Beryx spp.), red blackspot seabream (Pagellus bogaraveo). However, it should be noted that in some cases no disease or contaminant data exists for these species. -

Summary of Greenland Shark (Somniosus Microcephalus) Catch
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE AUTHOR(S) Northwest Atlantic Fisheries Organization Serial No. N6831 NAFO SCR Doc. 18/041 SCIENTIFIC COUNCIL MEETING – JUNE 2018 Summary of Greenland Shark (Somniosus microcephalus) catch in Greenland Halibut (Reinhardtius hippoglossoides) fisheries and scientific surveys conducted in NAFO Subarea 0 J.L. Bryk, K.J. Hedges and M.A. Treble Fisheries and Oceans Canada, Freshwater Institute, 501 University Cres., Winnipeg, Manitoba, Canada R3T 2N6 Abstract Understanding the impacts that fisheries have on the Greenland Shark, as a bycatch species, is important given the species is long-lived, slow growing and slow reproducing. These life history traits as well as its high trophic position make the Greenland Shark particularly vulnerable to exploitation. This report examines Greenland Shark catch data from Fisheries and Oceans Canada multi-species surveys conducted in NAFO Subarea 0 from 2004-2017, Northern Shrimp Research Foundation surveys conducted southeast of Baffin Island from 2005-2017, and at-sea commercial fishery observer data from the Subarea 0 Greenland Halibut fishery. The percent of sets with Greenland Shark in the offshore survey dataset ranged from 0% to 3.67% (comprised of 92 individuals). Length and weight data were available for most of the sharks caught with length ranging from 81 cm to 364 cm and weight from 5 kg to 600 kg. The percent of sets with Greenland Shark in the inshore surveys ranged from 0% to 276% (comprised of 127 individuals). Lengths varied from 100 cm to 400 cm. Greenland Shark bycatch occurs throughout the range of inshore and offshore commercial fisheries off the coast of Baffin Island. -
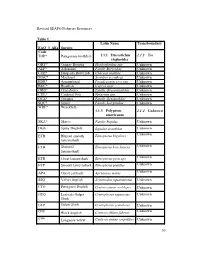
Revised SEAFO Fisheries Resources Table 1. . FAO 3 Alfa Species Code
Revised SEAFO Fisheries Resources Table 1. Latin Name Transboundary FAO 3 Alfa Species Code TOP* Patagonian toothfish 1.1.1 Dissostichus 1.1.2 Yes eleginoides ORY* Orange Roughy Hoplosthethus spp Unknown ALF* Alfonsino Family Berycidae Unknown CGE* Deep-sea Red Crab Chaceon maritae Unknown MAC* Mackerel Scomber scombrus Unknown EDR* Armourhead Pseudopentaceros spp. Unknown BOC* Boarfish Capros aper Unknown ORD* Oreo dories Family Oreosomatidae Unknown CDL* Cardinal Fish Epigonus spp. Unknown OCZ* Octopus Family Octopodidae Unknown SQC* Squid Family Loliginidae Unknown WRF* Wreckfish 1.1.3 Polyprion 1.1.4 Unknown americanus SKA* Skates Family Rajidae Unknown DGS Spiny Dogfish Squalus acanthias Unknown Unknown ETB Blurred smooth Etmopterus bigelowi lanternshark ETH Shorttail Etmopterus brachyurus Unknown lanternshark ETR Great lanternshark Etmopterus princeps Unknown ETP Smooth lanternshark Etmopterus pusillus Unknown Unknown APA Ghost catshark Apristurus manis SSQ Velvet dogfish Scymnodon squamulosus Unknown CYO Portuguese Dogfish Centroscymnus coelolepis Unknown GUQ Leafscale Gulper Centrophorus squamosus Unknown Shark GUP Gulper Shark Centrophorus granulosus Unknown CFB ǂ Black dogfish Centroscyllium fabricii Unknown CYP ǂ Longnose velvet Centroscymnus crepidater Unknown 30 dogfish CYY Unknown ǂ Shortnose velvet Centroscymnus dogfish cryptacanthus SCK ǂ Kitefin shark Dalatias licha Unknown ETE ǂ Etmopterus compagnoi Unknown ETI ǂ Broadbanded Etmopterus gracilispinis Unknown lanternshark ETM ǂ Unknown Southern Etmopterus granulosus lanternshark