Mark Report Satellite Tags (Mrpats) to Detail Large-Scale Horizontal Movements of Deep Water Species: First Results for the Greenland Shark (Somniosus Microcephalus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Greenland Shark (Somniosus Microcephalus)
Greenland shark (Somniosus microcephalus) feeding behavior on static fishing gear, effect of SMART (Selective Magnetic and Repellent-Treated) hook deterrent technology, and factors influencing entanglement in bottom longlines Scott M. Grant1, Rennie Sullivan1 and Kevin J. Hedges2 1 Centre for Sustainable Aquatic Resources, Memorial University of Newfoundland, St. John’s, NL, Canada 2 Central and Arctic Region, Arctic Aquatic Research Division, Fisheries and Oceans Canada, Winnipeg, MB, Canada ABSTRACT The Greenland Shark (Somniosus microcephalus) is the most common bycatch in the Greenland halibut (Reinhardtius hippoglossoides) bottom longline fishery in Cumberland Sound, Canada. Historically, this inshore fishery has been prosecuted through the ice during winter but winter storms and unpredictable landfast ice conditions since the mid-1990s have led to interest in developing a summer fishery during the ice-free season. However, bycatch of Greenland shark was found to increase substantially with 570 sharks captured during an experimental Greenland halibut summer fishery (i.e., mean of 6.3 sharks per 1,000 hooks set) and mortality was reported to be about 50% due in part to fishers killing sharks that were severely entangled in longline gear. This study investigated whether the SMART (Selective Magnetic and Repellent-Treated) hook technology is a practical deterrent to Greenland shark predation and subsequent bycatch on bottom longlines. Greenland Submitted 1 December 2017 shark feeding behavior, feeding kinematics, and variables affecting entanglement/ 22 April 2018 Accepted disentanglement and release are also described. The SMART hook failed to deter Published 17 May 2018 Greenland shark predation, i.e., all sharks were captured on SMART hooks, some Corresponding author Scott M. -

First Record of Swimming Speed of the Pacific Sleeper Shark Somniosus
Journal of the Marine First record of swimming speed of the Pacific Biological Association of the United Kingdom sleeper shark Somniosus pacificus using a baited camera array cambridge.org/mbi Yoshihiro Fujiwara , Yasuyuki Matsumoto, Takumi Sato, Masaru Kawato and Shinji Tsuchida Original Article Research Institute for Global Change (RIGC), Japan Agency for Marine-Earth Science and Technology (JAMSTEC), 2-15 Yokosuka, Kanagawa 237-0061, Japan Cite this article: Fujiwara Y, Matsumoto Y, Sato T, Kawato M, Tsuchida S (2021). First record of swimming speed of the Pacific Abstract sleeper shark Somniosus pacificus using a baited camera array. Journal of the Marine The Pacific sleeper shark Somniosus pacificus is one of the largest predators in deep Suruga Biological Association of the United Kingdom Bay, Japan. A single individual of the sleeper shark (female, ∼300 cm in total length) was 101, 457–464. https://doi.org/10.1017/ observed with two baited camera systems deployed simultaneously on the deep seafloor in S0025315421000321 the bay. The first arrival was recorded 43 min after the deployment of camera #1 on 21 July 2016 at a depth of 609 m. The shark had several remarkable features, including the Received: 26 July 2020 Revised: 14 April 2021 snout tangled in a broken fishing line, two torn anteriormost left-gill septums, and a parasitic Accepted: 14 April 2021 copepod attached to each eye. The same individual appeared at camera #2, which was First published online: 18 May 2021 deployed at a depth of 603 m, ∼37 min after it disappeared from camera #1 view. Finally, the same shark returned to camera #1 ∼31 min after leaving camera #2. -

Greenland Shark in the Barents Sea: Biology, Distribution and Bycatch
ICES CM 2010/E:26 Greenland shark in the Barents Sea: biology, distribution and bycatch by Rusyaev S.M., Sokolov K.M. and Drevetnyak K.V. E-mail:[email protected] Knipovich Polar Research Institute of Marine Fisheries and Oceanography (PINRO) 6 Knipovich Street, Murmansk, 183038 Russia Introduction Russian fishery for the Greenland shark (Somniosus microcephalus) was commenced in the Barents Sea in the late 19th century (Danilevskiy, 1862) and it was developed there in the early 20th century (Zhilinsky, 1923). Due to the diminishing of the demand for the liver fat of sharks that was caused by the commencement of the synthetic vitamin A production as well as since the coastal fishery was replaced by the oceanic one, the target fishery of the Greenland shark had been virtually ceased by 1961. In the absence of shark fishery, the research on distribution and abundance dynamics of a large predator may be important and urgent in the scope of ecosystem approach which is being developed in fishery science. The research on the Pacific Sleeper shark (Somniosus pacificus) showed that the abundance of representatives from that genus could significantly increase for a short time (Courtney, Sigler, 2002) and pose a threat for fisheries of the main commercial species (Orlov, 2003). Materials and methods Since there are no purposeful researches, the information on shark captures is limited and occasional, as a rule. The material for paper was the data on bycatches of Greenland shark by bottom and midwater gear in the Barents Sea obtained by research and fishing vessels: 233 captures from1981 to the present and 964 captures from January 1968 to August 2009, respectively. -

Origins of the Greenland Shark (Somniosus Microcephalus): Impacts of Ice‐Olation and Introgression
Received: 10 February 2017 | Revised: 7 June 2017 | Accepted: 21 July 2017 DOI: 10.1002/ece3.3325 ORIGINAL RESEARCH Origins of the Greenland shark (Somniosus microcephalus): Impacts of ice- olation and introgression Ryan P. Walter1,2 | Denis Roy3 | Nigel E. Hussey4 | Björn Stelbrink5 | Kit M. Kovacs6 | Christian Lydersen6 | Bailey C. McMeans2,7 | Jörundur Svavarsson8 | Steven T. Kessel9 | Sebastián Biton Porsmoguer10 | Sharon Wildes11 | Cindy A. Tribuzio11 | Steven E. Campana8 | Stephen D. Petersen12 | R. Dean Grubbs13 | Daniel D. Heath2 | Kevin J. Hedges14 | Aaron T. Fisk2 1Department of Biological Science, California State University, Fullerton, CA, USA 2Great Lakes Institute for Environmental Research, University of Windsor, Windsor, ON, Canada 3Department of Natural Resources and the Environment, Wildlife and Fisheries Conservation Center and Center for Environmental Sciences and Engineering, University of Connecticut, Storrs, CT, USA 4Biological Sciences, University of Windsor, Windsor, ON, Canada 5Justus Liebig University, Giessen, Germany 6Fram Centre, Norwegian Polar Institute, Tromsø, Norway 7Department of Biology, University of Toronto Mississauga, Mississauga, ON, Canada 8Faculty of Life and Environmental Sciences, University of Iceland, Reykjavík, Iceland 9Department of Fisheries and Wildlife, Michigan State University, East Lansing, MI, USA 10Mediterranean Institute of Oceanography (MIO) UM 110, Aix-Marseille University, CNRS/INSU, Toulon University, IRD, Marseille, France 11Auke Bay Laboratories, AFSC/NMFS/NOAA/DOC, Ted Stevens Marine Research Institute, Juneau, AK, USA 12Conservation and Research Department, Assiniboine Park Zoo, Winnipeg, MB, Canada 13Coastal and Marine Laboratory, Florida State University, St. Teresa, FL, USA 14Arctic Aquatic Research Division, Fisheries and Oceans Canada, Winnipeg, MB, Canada Correspondence Ryan P. Walter, Department of Biological Abstract Science, California State University, Fullerton, Herein, we use genetic data from 277 sleeper sharks to perform coalescent- based CA, USA. -

Greenland Shark (Somniosus Microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift
Greenland Shark (Somniosus microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift Nielsen, Julius; Christiansen, Jørgen Schou; Grønkjær, Peter; Bushnell, Peter; Steffensen, John Fleng; Kiilerich, Helene Overgaard; Præbel, Kim; Hedeholm, Rasmus Published in: Frontiers in Marine Science DOI: 10.3389/fmars.2019.00125 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Nielsen, J., Christiansen, J. S., Grønkjær, P., Bushnell, P., Steffensen, J. F., Kiilerich, H. O., ... Hedeholm, R. (2019). Greenland Shark (Somniosus microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift. Frontiers in Marine Science, 6, 1-11. [125]. https://doi.org/10.3389/fmars.2019.00125 Download date: 09. Apr. 2020 fmars-06-00125 April 2, 2019 Time: 17:28 # 1 ORIGINAL RESEARCH published: 04 April 2019 doi: 10.3389/fmars.2019.00125 Greenland Shark (Somniosus microcephalus) Stomach Contents and Stable Isotope Values Reveal an Ontogenetic Dietary Shift Julius Nielsen1,2,3,4*, Jørgen Schou Christiansen4,5, Peter Grønkjær6, Peter Bushnell7, John Fleng Steffensen1, Helene Overgaard Kiilerich6, Kim Præbel8 and Rasmus Hedeholm2 1 Marine Biological Section, University of Copenhagen, Helsingør, Denmark, 2 Greenland Institute of Natural Resources, Nuuk, Greenland, 3 Den Blå Planet, National Aquarium Denmark, Kastrup, Denmark, 4 Department of Arctic and Marine Biology, UiT The Arctic University of Norway, -

First Record of Swimming Speed of the Pacific Sleeper Shark Somniosus
Journal of the Marine First record of swimming speed of the Pacific Biological Association of the United Kingdom sleeper shark Somniosus pacificus using a baited camera array cambridge.org/mbi Yoshihiro Fujiwara , Yasuyuki Matsumoto, Takumi Sato, Masaru Kawato and Shinji Tsuchida Original Article Research Institute for Global Change (RIGC), Japan Agency for Marine-Earth Science and Technology (JAMSTEC), 2-15 Yokosuka, Kanagawa 237-0061, Japan Cite this article: Fujiwara Y, Matsumoto Y, Sato T, Kawato M, Tsuchida S (2021). First record of swimming speed of the Pacific Abstract sleeper shark Somniosus pacificus using a baited camera array. Journal of the Marine The Pacific sleeper shark Somniosus pacificus is one of the largest predators in deep Suruga Biological Association of the United Kingdom Bay, Japan. A single individual of the sleeper shark (female, ∼300 cm in total length) was 101, 457–464. https://doi.org/10.1017/ observed with two baited camera systems deployed simultaneously on the deep seafloor in S0025315421000321 the bay. The first arrival was recorded 43 min after the deployment of camera #1 on 21 July 2016 at a depth of 609 m. The shark had several remarkable features, including the Received: 26 July 2020 Revised: 14 April 2021 snout tangled in a broken fishing line, two torn anteriormost left-gill septums, and a parasitic Accepted: 14 April 2021 copepod attached to each eye. The same individual appeared at camera #2, which was First published online: 18 May 2021 deployed at a depth of 603 m, ∼37 min after it disappeared from camera #1 view. Finally, the same shark returned to camera #1 ∼31 min after leaving camera #2. -

Summary of Greenland Shark (Somniosus Microcephalus) Catch
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE AUTHOR(S) Northwest Atlantic Fisheries Organization Serial No. N6831 NAFO SCR Doc. 18/041 SCIENTIFIC COUNCIL MEETING – JUNE 2018 Summary of Greenland Shark (Somniosus microcephalus) catch in Greenland Halibut (Reinhardtius hippoglossoides) fisheries and scientific surveys conducted in NAFO Subarea 0 J.L. Bryk, K.J. Hedges and M.A. Treble Fisheries and Oceans Canada, Freshwater Institute, 501 University Cres., Winnipeg, Manitoba, Canada R3T 2N6 Abstract Understanding the impacts that fisheries have on the Greenland Shark, as a bycatch species, is important given the species is long-lived, slow growing and slow reproducing. These life history traits as well as its high trophic position make the Greenland Shark particularly vulnerable to exploitation. This report examines Greenland Shark catch data from Fisheries and Oceans Canada multi-species surveys conducted in NAFO Subarea 0 from 2004-2017, Northern Shrimp Research Foundation surveys conducted southeast of Baffin Island from 2005-2017, and at-sea commercial fishery observer data from the Subarea 0 Greenland Halibut fishery. The percent of sets with Greenland Shark in the offshore survey dataset ranged from 0% to 3.67% (comprised of 92 individuals). Length and weight data were available for most of the sharks caught with length ranging from 81 cm to 364 cm and weight from 5 kg to 600 kg. The percent of sets with Greenland Shark in the inshore surveys ranged from 0% to 276% (comprised of 127 individuals). Lengths varied from 100 cm to 400 cm. Greenland Shark bycatch occurs throughout the range of inshore and offshore commercial fisheries off the coast of Baffin Island. -
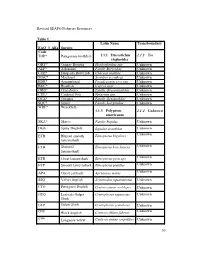
Revised SEAFO Fisheries Resources Table 1. . FAO 3 Alfa Species Code
Revised SEAFO Fisheries Resources Table 1. Latin Name Transboundary FAO 3 Alfa Species Code TOP* Patagonian toothfish 1.1.1 Dissostichus 1.1.2 Yes eleginoides ORY* Orange Roughy Hoplosthethus spp Unknown ALF* Alfonsino Family Berycidae Unknown CGE* Deep-sea Red Crab Chaceon maritae Unknown MAC* Mackerel Scomber scombrus Unknown EDR* Armourhead Pseudopentaceros spp. Unknown BOC* Boarfish Capros aper Unknown ORD* Oreo dories Family Oreosomatidae Unknown CDL* Cardinal Fish Epigonus spp. Unknown OCZ* Octopus Family Octopodidae Unknown SQC* Squid Family Loliginidae Unknown WRF* Wreckfish 1.1.3 Polyprion 1.1.4 Unknown americanus SKA* Skates Family Rajidae Unknown DGS Spiny Dogfish Squalus acanthias Unknown Unknown ETB Blurred smooth Etmopterus bigelowi lanternshark ETH Shorttail Etmopterus brachyurus Unknown lanternshark ETR Great lanternshark Etmopterus princeps Unknown ETP Smooth lanternshark Etmopterus pusillus Unknown Unknown APA Ghost catshark Apristurus manis SSQ Velvet dogfish Scymnodon squamulosus Unknown CYO Portuguese Dogfish Centroscymnus coelolepis Unknown GUQ Leafscale Gulper Centrophorus squamosus Unknown Shark GUP Gulper Shark Centrophorus granulosus Unknown CFB ǂ Black dogfish Centroscyllium fabricii Unknown CYP ǂ Longnose velvet Centroscymnus crepidater Unknown 30 dogfish CYY Unknown ǂ Shortnose velvet Centroscymnus dogfish cryptacanthus SCK ǂ Kitefin shark Dalatias licha Unknown ETE ǂ Etmopterus compagnoi Unknown ETI ǂ Broadbanded Etmopterus gracilispinis Unknown lanternshark ETM ǂ Unknown Southern Etmopterus granulosus lanternshark -

First Record of Swimming Speed of the Pacific Sleeper Shark Somniosus
Journal of the Marine First record of swimming speed of the Pacific Biological Association of the United Kingdom sleeper shark Somniosus pacificus using a baited camera array cambridge.org/mbi Yoshihiro Fujiwara , Yasuyuki Matsumoto, Takumi Sato, Masaru Kawato and Shinji Tsuchida Original Article Research Institute for Global Change (RIGC), Japan Agency for Marine-Earth Science and Technology (JAMSTEC), 2-15 Yokosuka, Kanagawa 237-0061, Japan Cite this article: Fujiwara Y, Matsumoto Y, Sato T, Kawato M, Tsuchida S (2021). First record of swimming speed of the Pacific Abstract sleeper shark Somniosus pacificus using a baited camera array. Journal of the Marine The Pacific sleeper shark Somniosus pacificus is one of the largest predators in deep Suruga Biological Association of the United Kingdom Bay, Japan. A single individual of the sleeper shark (female, ∼300 cm in total length) was 101, 457–464. https://doi.org/10.1017/ observed with two baited camera systems deployed simultaneously on the deep seafloor in S0025315421000321 the bay. The first arrival was recorded 43 min after the deployment of camera #1 on 21 July 2016 at a depth of 609 m. The shark had several remarkable features, including the Received: 26 July 2020 Revised: 14 April 2021 snout tangled in a broken fishing line, two torn anteriormost left-gill septums, and a parasitic Accepted: 14 April 2021 copepod attached to each eye. The same individual appeared at camera #2, which was First published online: 18 May 2021 deployed at a depth of 603 m, ∼37 min after it disappeared from camera #1 view. Finally, the same shark returned to camera #1 ∼31 min after leaving camera #2. -

SHARK FACTS There Are 510 Species of Sharks
1 SHARK FACTS There are 510 species of sharks. Let’s learn more about a few of them. Common Six-gilled Thresher Shark Shark • Known for its 10 foot tail • Can grow up to 16 feet long • Stuns and herds fish with its long tail • Has six pairs of gills instead of the average of five • Warm blooded • Has one dorsal fin at the back of its body • Feeds on squid and schooling fish • Also known as cow shark or mud shark • Prefers to stay towards the top of deep bodies • Deep water shark of water Shortfin Great Mako Hammerhead Shark Shark • Bluish gray on top part of body and white on • Eyes are at opposite sides of its rectangular the belly shaped head • Has extremely sharp teeth, that stick out even when • Feeds on crustaceans, octopuses, rays and its mouth is shut small sharks • Feeds on sharks, swordfish and tuna • Usually found around tropical reefs • Jumps high in the air to escape fishing hooks • Can give birth to over 40 pups in one litter • Fastest of all the sharks as it can swim over 30 mph • Has a heigtened sense of electro-reception 2 SHARK FACTS Bull Nurse Shark Shark • Can grow up to 11 feet long and over 200 pounds • Has long, fleshy appendages called barbels that hang below its snout • Gray to brown in color with a white belly • Feeds on crab, lobster, urchins and fish • Feeds on fish, dolphins, sea turtles and other sharks • Usually found near rocky reefs, mudflats • Found in fresh and salt water and sandbars • Aggressive species • Enjoys laying on the ocean floor • Nocturnal animal Great Epaulette White Shark Shark • Can grow -

Greenland Shark (Somniosus Microcephalus) Catches in Fisheries Conducted in the Northwest Atlantic Fisheries Organization Regulatory Area
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE AUTHOR(S) Northwest Atlantic Fisheries Organization Serial No. N6804 NAFO SCR Doc. 18/020REV2 SCIENTIFIC COUNCIL MEETING – JUNE 2018 Greenland shark (Somniosus microcephalus) catches in fisheries conducted in the Northwest Atlantic Fisheries Organization Regulatory Area by Lisa C. Hendrickson1, Jana Aker2, Sebastian Glindtvad2 and Tom Blasdale2 1U. S. National Marine Fisheries Service, Northeast Fisheries Science Center, 166 Water St., Woods Hole, MA 02543 2Northwest Atlantic Fisheries Organization, Morris Drive, Suite 100, Dartmouth, Nova Scotia, Canada B3B 1K8 Abstract The Greenland shark (Somniosus microcephalus), a large, benthopelagic shark, has been listed as “Near Threatened” on the International Union for the Conservation of Nature and Natural Resources (IUCN) Red List since 2006. The IUCN-recommended conservation actions include documentation of catches in North Atlantic and Arctic fisheries and determination of fisheries- related population declines. This report is a response, in part, to a request from the Fisheries Commission of the Northwest Atlantic Fisheries Organization (NAFO) to the Scientific Council for documentation of Greenland shark catches in NAFO fisheries and summarization of existing data useful for assessing the status of the population inhabiting NAFO waters. We found that nominal catches of Greenland shark have been reported to NAFO since 2002, but reporting was inconsistent. Catches ranged from 1 t in 2002 and 2004 to 71 t in 2017; 69% of the catches during 2002-2017 were from Subarea 1. NAFO Observer Program data indicated that bycatch of Greenland shark in the NAFO Regulatory Area increased rapidly between 2014 and 2017, from 34 t to 281 t and from at least 75 to 180 individuals, respectively. -

Archival Pop-Off Tag Tracking of Greenland Sharks Somniosus Microcephalus in the High Arctic Waters of Svalbard, Norway
Vol. 468: 255–265, 2012 MARINE ECOLOGY PROGRESS SERIES Published November 14 doi: 10.3354/meps09962 Mar Ecol Prog Ser Archival pop-off tag tracking of Greenland sharks Somniosus microcephalus in the High Arctic waters of Svalbard, Norway Aaron T. Fisk1,*, Christian Lydersen2, Kit M. Kovacs2 1University of Windsor, Great Lakes Institute for Environmental Research, 401 Sunset Avenue, Windsor, Ontario N9B 3P4, Canada 2Norwegian Polar Institute, Fram Centre, Tromsø 9296, Norway ABSTRACT: The Greenland shark Somniosus microcephalus is the largest fish in Arctic waters and a significant predator that is likely numerous, but little is known about its movement patterns or habitat preferences. In June 2008 and 2009, 20 archival pop-off tags were deployed on Green- land sharks in coastal waters in Svalbard, Norway (~80° N), to address this knowledge gap. Over the period June to December, 14 of the tags reported data. The swimming depth of the sharks was 111 ± 74 m (mean ± SD), but they occupied waters from the surface down to a depth of at least 1560 m (the depth limit of the tags), which is the deepest confirmed record for this species. The sharks displayed a wide range of depths within each 6 h time bin collected by the tags, with no obvious diel movement pattern. They experienced temperatures from −1.5 to 7.4°C (mean ± SD = 3.8 ± 1.4°C). The average depth of swimming increased and temperatures experienced by the sharks decreased from June through December. Most individuals moved north from the tagging area, and travelled a range of distances during their variable deployment times, but most tags popped off <500 km from the tagging site.