Best Practices for Treating Ammonia in Landfill Leachate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Making Decisions About Water and Wastewater for Aqueous Operation
Making Decisions about Water and Wastewater for Aqueous Operation John F. Russo Chapter 2.17 Handbook for Critical Cleaning Editor-in-Chief Barbara Kanegsberg Reprinted with permission from CRC Press www.crcpress.com INTRODUCTION..................................................................................................................................3 TYPICAL CLEANING SYSTEM............................................................................................................3 OPERATIONAL SITUATIONS OF TYPICAL USER ...............................................................................4 Determining the Water Purity Requirements .........................................................................................4 Undissolved Contaminants............................................................................................................4 Dissolved Contaminants...............................................................................................................4 Undissolved and Dissolved Contaminants........................................................................................5 Other Conditions...........................................................................................................................5 Determining the Wastewater Volume Produced .....................................................................................6 Source Water Trea tment .....................................................................................................................6 No -

A Combined Vermifiltration-Hydroponic System
applied sciences Article A Combined Vermifiltration-Hydroponic System for Swine Wastewater Treatment Kirill Ispolnov 1,*, Luis M. I. Aires 1,Nídia D. Lourenço 2 and Judite S. Vieira 1 1 Laboratory of Separation and Reaction Engineering-Laboratory of Catalysis and Materials (LSRE-LCM), School of Technology and Management (ESTG), Polytechnic Institute of Leiria, 2411-901 Leiria, Portugal; [email protected] (L.M.I.A.); [email protected] (J.S.V.) 2 Applied Molecular Biosciences Unit (UCIBIO)-REQUIMTE, Department of Chemistry, NOVA School of Science and Technology (FCT), NOVA University of Lisbon, 2829-516 Caparica, Portugal; [email protected] * Correspondence: [email protected] Abstract: Intensive swine farming causes strong local environmental impacts by generating ef- fluents rich in solids, organic matter, nitrogen, phosphorus, and pathogenic bacteria. Insufficient treatment of hog farm effluents has been reported for common technologies, and vermifiltration is considered a promising treatment alternative that, however, requires additional processes to remove nitrate and phosphorus. This work aimed to study the use of vermifiltration with a downstream hydroponic culture to treat hog farm effluents. A treatment system comprising a vermifilter and a downstream deep-water culture hydroponic unit was built. The treated effluent was reused to dilute raw wastewater. Electrical conductivity, pH, and changes in BOD5, ammonia, nitrite, nitrate, phosphorus, and coliform bacteria were assessed. Plants were monitored throughout the experiment. Electrical conductivity increased due to vermifiltration; pH stayed within a neutral to mild alkaline range. Vermifiltration removed 83% of BOD5, 99% of ammonia and nitrite, and increased nitrate by Citation: Ispolnov, K.; Aires, L.M.I.; 11%. -

Reuse: a Safe and Effective Way to Save Water
SOUTH FLORIDA WATER MANAGEMENT DISTRICT Water Reuse: A safe and effective way to save water ON THE INSIDE The demand for water is projected to increase over the long term in n Reclaimed water and reuse explained South Florida. Urban populations, agricultural operations and the environment depend on adequate water supplies. Fresh ground n Reuse success stories water and surface water will not be sufficient to satisfy all future n Reuse on a regional level demands. Meeting this growing thirst hinges on efforts to develop How water is reclaimed n alternative water sources. This brochure looks at one of the ways to n Purple pipes signify conserve Florida’s water resources — reclaiming water for reuse. reclaimed water Consider what happens to the water used inside the home. Once down the drain, this water is piped to the local wastewater treatment plant where it undergoes treatment to meet state standards for disposal. Historically, most of the water was disposed by injecting it deep underground or by discharging to surrounding waters or to the ocean. This is a wasteful way to treat such a valuable resource. More and more communities are finding that wastewater need not be wasted. They are reclaiming this water for irrigation of residential lots, golf courses, sports fields and orange groves; industrial cooling; car washing; fire protection; and Reclaimed water sign in Collier County groundwater recharge. Reuse is also beneficial to the environment. Success Stories During times of drought, reclaimed water •Pompano Beach – The city takes is a dependable source of water because wastewater being piped to the ocean, its availability is not dependent on rainfall. -

Wastewater Treatment: Overview and Background
Wastewater Treatment: Overview and Background Claudia Copeland Specialist in Resources and Environmental Policy October 30, 2014 Congressional Research Service 7-5700 www.crs.gov 98-323 Wastewater Treatment: Overview and Background Summary The Clean Water Act prescribes performance levels to be attained by municipal sewage treatment plants in order to prevent the discharge of harmful wastes into surface waters. The act also provides financial assistance so that communities can construct treatment facilities to comply with the law. The availability of funding for this purpose continues to be a major concern of states and local governments. This report provides background on municipal wastewater treatment issues, federal treatment requirements and funding, and recent legislative activity. Meeting the nation’s wastewater infrastructure needs efficiently and effectively is likely to remain an issue of considerable interest to policymakers. Congressional Research Service Wastewater Treatment: Overview and Background Contents Introduction ...................................................................................................................................... 1 Federal Aid for Wastewater Treatment ............................................................................................ 1 How the SRF Works .................................................................................................................. 2 Other Federal Assistance .......................................................................................................... -

Ion Exchange and Disposal Issues Associated with the Brine Waste Stream
FWRJ Ion Exchange and Disposal Issues Associated With the Brine Waste Stream Julie Karleskint, Daniel Schmidt, Robert Anderson, Jayson Page, and A.J. Berndt he City of Arcadia recently completed from the local water supply authority, it was Julie Karleskint, P.E., is a senior construction of a new 1.5-mil-gal-per- determined that ion exchange would be the associate with Hazen and Sawyer in Tday (mgd) water treatment plant most cost-effective option for construction. A Sarasota. Daniel Schmidt, P.E., is a (WTP) using ion exchange technology to re- reduction in capacity was also provided since senior associate with Hazen and Sawyer place its 3-mgd lime softening WTP. The lime the City’s water supply source, groundwater in Tampa. Robert Anderson, P.E., is a plant had reached the end of its serviceable life from the intermediate aquifer, was limited senior associate with Hazen and Sawyer and the treatment of groundwater for the re- based on current pumping limitations and in Orlando. Jayson Page, P.E., is a moval of radionuclides, hardness, sulfides, or- permitted capacity. senior associate with Hazen and Sawyer ganic carbon, and fluoride was desired in The groundwater is supplied from six order to provide safe drinking water to the wells, approximately 350 ft deep and located in Coral Gables. A.J. Berndt is the utility community. After evaluating several treatment within a 1-mi radius of the plant. A summary director for the City of Arcadia. technologies, including lime treatment, of the water quality from the wellfield is shown nanofiltration, ion exchange, and purchases in Table 1. -

Review of the Ion Exchange Filtration Process and Materials Used August 4, 2020
National Organic Standards Board Handli ng Subcommittee Proposal Review of the Ion Exchange Filtration Process and Materials Used August 4, 2020 Background: In an August 27, 2019, memo the National Organic Program requested the NOSB provide recommendations related to the process of ion exchange filtration in the handling of organic products. It has become clear that there is inconsistency between certifiers in how they approve or disapprove this type of process. Some certifiers require only the solutions that are used to recharge the ion exchange membranes be on the National List at § 205.605. Others require that all materials, including ion exchange membranes and resins be on the National List. The National Organic Program provided clarification to certifying agents in an email sent on May 7, 2019, that nonagricultural substances used in the ion-exchange process must be present on the National List. This would include, but is not limited to, resins, membranes and recharge materials. Originally, the NOP asked all operations to come into compliance with the statement above by May 1, 2020. However, in response to requests for clarification of NOP’s rationale, as well as requests to extend the timeline for implementation, the NOP delayed the implementation date in order to gather more information and requested that NOSB review the issue. Manufacturers and certifiers who wish to continue allowance of the ion exchange process disagree with some of the findings of the NOP on this complex issue. The different opinions of the need for resins, recharge materials and membranes to be present on the National List, as well as how they interact with each other and the liquid run through the process, is complicated and the NOP therefore asked the NOSB to take on this issue. -

Community Wastewater Treatment by Using Vermifiltration Technique
International Journal of Engineering Research and Technology. ISSN 0974-3154 Volume 10, Number 1 (2017) © International Research Publication House http://www.irphouse.com Community Wastewater Treatment By Using Vermifiltration Technique Author 1 Nandini Misal Assistant Professor,Department of Civil Engineering,D.Y .Patil College of Engineering and Technology, KasabaBawada,Kolhapur Author 2 Mr.NitishA.Mohite Assistant Professor,Department of Civil Engineering,D.Y .Patil College of Engineering and Technology, KasabaBawada,Kolhapur Abstract cowdung,clay and loaded with vermis-Eisenia fetida Now-a-days many developing countries cannot afford the earthworms. wastewater treatment processes as they are costly,need more The wastewater is allowed to pass through the filter,the space to construct the treatment plant and in addition use of earthworms consume and metabolise oils,fats and other chemicals for the treatment.They need some more options at compounds.The water percolating through is collected in low cost,space saving and ecofriendly another container.Earlier report of Sinha et.al(2008) have techniques.Vermifiltration is one of the simple,low proved that the body of earthworms works as a “biofilter”and cost,ecofriendly,chemical free technique used to treat the the body walls absorbs the solids from wastewater.It has been canteen wastewater using the Eisenia fetida earthworm observed that the earthworms are potentially capable of species.The earthworms are potentially capable of digesting digesting the waste organic material and remove the 5days the waste organic material and reduce it through ingestion.It is BOD5 near about 90%,COD by 85-90%, TS by 90-95%,TDS considered to be an innovative ecofriendly technology that by 95%,TSS by 95-98%. -

Reverse Osmosis As a Pretreatment of Ion Exchange Equipment at PSE
Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. Please Note: The author retains the copyright while the New Jersey Institute of Technology reserves the right to distribute this thesis or dissertation Printing note: If you do not wish to print this page, then select “Pages from: first page # to: last page #” on the print dialog screen The Van Houten library has removed some of the personal information and all signatures from the approval page and biographical sketches of theses and dissertations in order to protect the identity of NJIT graduates and faculty. ABSTRACT REVERSE OSMOSIS AS A PRETREATMENT FOR ION EXCHANGE AT PSE&G'S HUDSON GENERATING STATION by Steven Leon Public Service Electric and Gas Company's Hudson Generating Station has historically had problems providing sufficient high quality water for its two once through, supercritical design boilers. The station requires over 60 million gallons annually to compensate for system losses. -

Glossary of Wastewater Terms
Glossary of Wastewater Terms Activated Sludge Sludge that has undergone flocculation forming a bacterial culture typically carried out in tanks. Can be extended with aeration. Advanced Primary Treatment The use of special additives to raw wastewater to cause flocculation or clumping to help settling before the primary treatment such as screening. Advanced Wastewater Treatment Any advanced process used above and beyond the defacto typical minimum primary and secondary wastewater treatment. Aerobic Wastewater Treatment Oxygen dependent wastewater treatment requiring the presence of oxygen for aerobic bacterial breakdown of waste. Alkalinity A measure of a substances ability to neutralize acid. Water containing carbonates, bicarbonates, hydroxides, and occasionally borates, silicates, and phosphates can be alkaline. Alkaline substances have a pH value over 7 Anaerobic Wastewater Treatment Wastewater treatment in the absence of oxygen, anaerobic bacteria breakdown waste. Bacteria Single cell microscopic living organisms lacking chlorophyll, which digest many organic and inorganic substances. An essential part of the ecosystem including within human beings. Bioengineering The use of living plants as part of the system, be it wastewater treatment, erosion control, water polishing, habitat repair and on. Biosolids Rich organic material leftover from aerobic wastewater treatment, essentially dewatered sludge that can be re-used. BOD - Biochemical Oxygen Demand Since oxygen is required in the breakdown or decomposition process of wastewater, its "demand" or BOD, is a measure of the concentration of organics in the wastewater. Clarifier A piece of wastewater treatment equipment used to "clarify" the wastewater, usually some sort of holding tank that allows settling. Used when solids have a specific gravity greater than 1. -

Wastewater Ion Exchange Services
WASTEWATER ION EXCHANGE SERVICES Evoqua owns and operates a fully permitted RCRA facility with the technical expertise Evoqua Water Technologies Wastewater Ion Exchange (WWIX) services and and equipment necessary to safely manage equipment help customers meet their wastewater handling challenges. Evoqua regeneration of the spent resins while provides system design, installation, and custom services that treat wastewater remaining environmentally compliant. contaminated with heavy metals. Zinc, copper, nickel and chromium are just a Where practical, the media/resin is recycled few examples of the metals that can be removed to low part per billion levels. Our for future use. We can accept both non- wastewater treatment systems are designed to meet your discharge requirements, hazardous and hazardous waste. achieve the water quality level needed for reuse and recycling and minimize the liability associated with on-site storage and handling of chemicals and wastes. Typical Applications Markets Every Application has Unique Requirements • Metal finishing, electroplating and Each application is examined to determine the system configuration that best coating meets current and future needs. The system components are selected based upon • Parts washing available manpower, space limitations, access limitation and the specific reuse or • Aerospace discharge quality required. If needs change, our wastewater treatment systems are • Anodizing flexible – we can simply change the media types and/or tank size saving our clients • Circuit board assembly and significant capital expense. manufacturing What is WWIX Service? • Microelectronics • EDM Ion exchange (IX) is a proven and cost-effective technology for removing inorganic • General manufacturing contaminants. Evoqua‘s WWIX service utilizes ion exchange resins and other • Groundwater remediation media selected to remove specific ionic contaminants from groundwater, industrial • Magnetic/digital media production wastewater, and process water for recycle. -
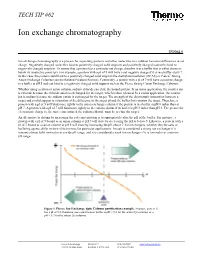
Ion Exchange Chromatography
TECH TIP #62 Ion exchange chromatography TR0062.0 Ion exchange chromatography is a process for separating proteins and other molecules in a solution based on differences in net charge. Negatively charged molecules bind to positively charged solid supports and positively charged molecules bind to negatively charged supports. To ensure that a protein has a particular net charge, dissolve it in a buffer that is either above or below its isoelectric point (pI). For example, a protein with a pI of 5 will have a net negative charge if it is in a buffer at pH 7. In this case, the protein could bind to a positively charged solid support like diethylaminoethanol (DEAE) or Pierce® Strong Anion Exchange Columns (see the Related Products Section). Conversely, a protein with a pI of 7 will have a positive charge in a buffer at pH 5 and can bind to a negatively charged solid support such as the Pierce Strong Cation Exchange Columns. Whether using a cation or anion column, sodium chloride can elute the bound protein. In an anion application, the counter ion is chloride because the chloride anion is exchanged for the target, which is then released. In a cation application, the counter ion is sodium because the sodium cation is exchanged for the target. The strength of the electrostatic interaction between a target and a solid support is a function of the difference in the target pI and the buffer that contains the target. Therefore, a protein with a pI of 5 will bind more tightly to the anion exchange column if the protein is in a buffer at pH 8 rather than at pH 7. -

The Procession of Constructed Wetland Removal Mechanism of Pollutants
4th International Conference on Mechanical Materials and Manufacturing Engineering (MMME 2016) The procession of constructed wetland removal mechanism of pollutants Ruqiong Qin, Hong Chen Guangxi Polytechnic of Construction, Nanning 530003, China ABSTRACT: Constructed wetland ecosystem is a complex project, and the removal mechanism is complicated. This article is mainly summary the degradation mechanism of pollutants from the main processing and removal method of wetland functions. Constructed wetland treatment effects on plant species, substrate, microorgan- isms, climate and other factors. KEYWORD: Constructed wetlands; removal mechanism; influencing factors 1 INTRODUCTION flow in a certain direction, the constructed wetland is If sewage collection and treatment improperly, the ru- primarily use of soil, the artificial medium, plants, ral ecological environment and living environment and physics, chemistry, biology of microorganisms will be damaged, then resulting in the pollution of triple synergy to treatment sewage, sludge. The mech- surface water and shallow groundwater. China is vig- anism of constructed wetland is adsorption, retention, orously promoting the construction of new rural, filtration, oxidation-reduction, precipitation, micro- however, the rural economic is under development bial decomposition, transformation, plant shelter, res- for a long-term, and the cultural quality of the resi- idue accumulation, water and nutrient uptake and dents is not high, moreover, capital, technology and transpiration the effect of various types of animals. talents are inadequate, sewage treatment technology How about the removal of wetland? We will ex- becomes more passive. For the sewage treatment planted the constructed wetland removal of organic characteristics of the rural point of view, it should be matter, nitrogen, phosphorus, heavy metals, microor- fully taken into account in financial, personnel, ganisms and other substances in detailed.