Development of Photocleavable Linker Groups for Application To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A-PDF Split DEMO : Purchase from to Remove the Watermark
A-PDF Split DEMO : Purchase from www.A-PDF.com to remove the watermark Figure 7.5 (a) Transition state for E2H-syn elimination. (b) Transition state for E2H-anti elimination. (c) Transition state for E,C elimination. tion state similar to that shown in Figure 7.5~is expected, since base attacks the molecule backside to the leaving group but frontside to the /3 hydrogen. Let us now turn to the experimental results to see if these predictions are borne out in fact. It has long been known that E2H reactions normally give preferentially anti elimination. For example, reaction of meso-stilbene dibromide with potassium ethoxide gives cis-bromostilbene (Reaction 7.39), whereas reaction of the D,L-dibromide gives the trans product (Reaction 7.40).lo2 A multitude of other examples exist-see, for example, note 64 (p. 355) and note 82 (p. 362). E2H reactions do, however, give syn elimination when: (1) an H-X di- hedral angle of 0" is achievable but one of 180" is not or, put another way, H and X can become syn-periplanar but not trans-periplanar ; (2) a syn hydrogen is much more reactive than the anti ones; (3) syn elimination is favored for steric reasons; and (4) an anionic base that remains coordinated with its cation, that, in turn, is coordinated with the leaving group, is used as catalyst. The very great importance of category 4 has only begun to be fully realized in the early 1970s. lo2 P. Pfeiffer, 2. Physik. Chem. Leibrig, 48, 40 (1904). 1,2-Elimination Reactions 371 An example of category 1 is found in the observation by Brown and Liu that eliminations from the rigid ring system 44, induced by the sodium salt of 2- cyclohexylcyclohexanol in triglyme, produces norborene (98 percent) but no 2-de~teronorbornene.~~~The dihedral angle between D and tosylate is O", but Crown ether present: No Yes that between H and tosylate is 120". -

Absolute Fluorescence Quantum Yields Using the FL 6500 Fluorescence Spectrometer
APPLICATION NOTE Fluorescence Spectroscopy Authors: Kathryn Lawson-Wood Steve Upstone Kieran Evans PerkinElmer, Inc. Seer Green, UK Absolute Fluorescence Quantum Yields Using the FL 6500 Introduction Fluorescence quantum yield Fluorescence Spectrometer (ΦF) is a characteristic property of a fluorescent species and is denoted as the ratio of the number of photons emitted through fluorescence, to the number of photons absorbed by the fluorophore (Equation 1). ΦF ultimately relates to the efficiency of the pathways leading to emission of fluorescence (Figure 1), providing the probability of the excited state being deactivated by fluorescence rather than by other competing relaxation processes. The magnitude of ΦF is directly related to the intensity of the observed fluorescence.1 Number of photons emitted through fluorescence ΦF = (1) Number of photons absorbed Fluorescence quantum yield can be measured using two methods: the relative method and the absolute method. In comparison with relative quantum yield measurements, the absolute method is applicable to both solid and solution phase measurements. The ΦF can be determined in a single measurement without the need for a reference standard. It is especially useful for samples which absorb and emit in wavelength regions for which there are no reliable quantum yield standards available.2 The importance of ΦF has been shown in several industries, standard. It is especially useful for samples which absorb and including the research, development and evaluation of audio emit in wavelength regions for which there are no reliable visual equipment, electroluminescent materials (OLED/LED) and quantum yield standards available. This method requires an fluorescent probes for biological assays.3 This application integrating sphere which allows the instrument to collect all of demonstrates the use of the PerkinElmer FL 6500 Fluorescence the photons emitted from the sample. -
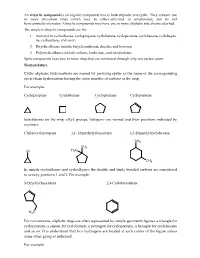
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

Studies on Fluorescence Efficiency and Photodegradation Of
Laser Chemistry, 2002, Vol. 20(2–4), pp. 99–110 Studies on Fluorescence Efficiency and Photodegradation of Rhodamine 6G Doped PMMA Using a Dual Beam Thermal Lens Technique ACHAMMA KURIAN*, NIBU A GEORGE, BINOY PAUL, V. P. N. NAMPOORI and C. P. G. VALLABHAN International School of Photonics, Cochin University of Science & Technology, Cochin 682022, India (Received11September 2002) In this paper we report the use of the dual beam thermal lens technique as a quantitative method to determine absolute fluorescence quantum efficiency and concentration quenching of fluorescence emission from rhodamine 6G doped Poly(methyl methacrylate) (PMMA), prepared with different concentrations of the dye. A comparison of the present data with that reported in the literature indicates that the observed variation of fluorescence quantum yield with respect to the dye concentration follows a similar profile as in the earlier reported observations on rhodamine 6G in solution. The photodegradation of the dye molecules under cw laser excitation is also studied using the present method. Key words: Thermal lens; Fluorescence quantum yield; Photostability; Dye doped polymer 1 INTRODUCTION From the mid 60s, dye lasers have been attractive sources of coherent tunable radiation because of their unique operational flexibility. The salient features of dye lasers are their tunability, with emission from near ultraviolet to the near infrared. High gain, broad spectral bandwidth enabled pulsed and continuous wave operation. Although dyes have been demonstrated to lase in the solid, liquid or gas phases, liquid solutions of dyes in suitable organic * Corresponding author. Catholicate College, Pathanamthitta 689645, India. E-mail: [email protected] 100 A. KURIAN et al. -

New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis
New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Author: Jason Joseph Marineau Persistent link: http://hdl.handle.net/2345/1741 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2010 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College The Graduate School of Arts and Sciences Department of Chemistry NEW APPLICATIONS OF CYCLOBUTADIENE CYCLOADDITIONS: DIVERSITY AND TARGET ORIENTED SYNTHESIS a dissertation by JASON JOSEPH MARINEAU submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2010 © Copyright by JASON JOSEPH MARINEAU 2010 New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Jason J. Marineau Thesis Advisor: Professor Marc. L. Snapper Abstract Cyclobutadiene cycloadditions provide rapid access to rigid polycyclic systems with high strain energy and unusual molecular geometries. Further functionalization of these systems allows entry into unexplored chemical space. A tricarbonylcyclobutadiene iron complex on solid support enables exploration of these cycloadditions in a parallel format amenable to diversity oriented synthesis. Modeling of the cycloaddition transition states with density functional calculations provides a theoretical basis for analysis of the regioselectivity observed in generation of these substituted bicyclo[2.2.0]hexene derivatives. The high strain energy accessible in cyclobutadiene cycloadducts and their derivatives renders them useful synthons for access to medium-ring natural products through ring expansion. Torilin, a guaiane sesquiterpene isolated from extracts of the fruits of Torilis japonica, exhibits a range of biological activities including testosterone 5 -reductase inhibition, hKv1.5 channel blocking, hepatoprotective, anti-inflammatory and anti-cancer effects. -

Here Science-Based Cost- Effective Pathways Forward?
Sponsors Institute of Atomic and Molecular Sciences, Academia Sinica, Taiwan PIRE-ECCI Program, UC Santa Barbara, USA Max-Planck-Gesellschaft, Germany National Science Council, Taiwan Organizing committee Dr. Kuei-Hsien Chen (IAMS, Academia Sinica, Taiwan) Dr. Susannah Scott (University of California - Santa Barbara, USA) Dr. Alec Wodtke (University of Göttingen & Max-Planck-Gesellschaft, Germany) Table of Contents General Information .............................................................................................. 1 Program for Sustainable Energy Workshop ....................................................... 3 I01 Dr. Alec M. Wodtke Beam-surface Scattering as a Probe of Chemical Reaction Dynamics at Interfaces ........................................................................................................... 7 I02 Dr. Kopin Liu Imaging the steric effects in polyatomic reactions ............................................. 9 I03 Dr. Chi-Kung Ni Energy Transfer of Highly Vibrationally Excited Molecules and Supercollisions ................................................................................................. 11 I04 Dr. Eckart Hasselbrink Energy Conversion from Catalytic Reactions to Hot Electrons in Thin Metal Heterostructures ............................................................................................... 13 I05 Dr. Jim Jr-Min Lin ClOOCl and Ozone Hole — A Catalytic Cycle that We Don’t Like ............... 15 I06 Dr. Trevor W. Hayton Nitric Oxide Reduction Mediated by a Nickel Complex -

Measurement of Fluorescence Quantum Yields on ISS Instrumentation Using Vinci Yevgen Povrozin and Ewald Terpetschnig
TECHNICAL NOTE Measurement of Fluorescence Quantum Yields on ISS Instrumentation Using Vinci Yevgen Povrozin and Ewald Terpetschnig Introduction The quantum yield Q can also be described by the relative rates of the radiative and non-radiative pathways, which deactivate the excited state: kr Q [1 ] kkr nr where and correspond to radiative and non-radiative processes, respectively. In this equation, kr knr knr describes the sum of the rate constants for the various processes that compete with the emission process. These processes include photochemical, and dissociative processes including other, less well characterized changes that result in a return to the ground state with simultaneous dissipation of the energy of the excited state into heat. These latter processes are collectively called non-radiative transitions and two types have been clearly recognized: intersystem crossing and internal conversion. Intersystem crossing related to the radiationless spin inversion of a singlet state (S1) in the excited state into a triplet state (T1). The luminescence quantum yield is also given as the ratio of the number of photons emitted to the number of photons absorbed by the sample: photonsem Q [ 2] photonsabs While measurements of the “absolute” quantum yield do require more sophisticated instrumentation [1], it is easier to determine the “relative” quantum yield of a fluorophore by comparing it to a standard with a known quantum yield. Some of the most common standards are listed in table below: Conditions for Quantum Yield [Q.Y.] Q.Y. Excitation Q.Y. Ref. Standards [nm] [%] Measurements Cy3 4 PBS 540 2 Cy5 27 PBS 620 2 Cresyl Violet 53 Methanol 580 3 1 ISS TECHNICAL NOTE Conditions for Quantum Yield [Q.Y.] Q.Y. -

Effect of Enzyme/Substrate Ratio on the Antioxidant Properties of Hydrolysed African Yam Bean
African Journal of Biotechnology Vol. 11(50), pp. 11086-11091, 21 June, 2012 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.2271 ISSN 1684–5315 ©2012 Academic Journals Full Length Research Paper Effect of enzyme/substrate ratio on the antioxidant properties of hydrolysed African yam bean Fasasi Olufunmilayo*, Oyebode Esther and Fagbamila Oluwatoyin Department of Food Science and Technology, P. M. B. 704, Federal University of Technology, Akure, Nigeria. Accepted 8 June, 2012 The use of natural antioxidant as compared with synthetic antioxidant in food processing is a growing trend as consumers prefer natural to synthetic antioxidant mainly on emotional ground. This study investigates the antioxidant activity of hydrolysed African yam bean (Sphenostylis sternocarpa) which is regarded as one of the neglected underutilized species (NUS) of crop in Africa and Nigeria especially to improve food security and boost the economic importance of the crop. The antioxidant properties of African yam bean hydrolysates (AYH) produced at different enzyme to substrate (E/S) ratios of 1: 100 and 3: 100 (W/V) using pepsin (pH 2.0, 37°C) were studied. 2, 2-Diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity of the hydrolysates was significantly influenced by the E\S ratio as DPPH radical scavenging activity ranged from 56.1 to 75.8% in AYH (1: 100) and 33.3 to 58.8% in AYH (3:100) with 1000 µg/ml having the highest DPPH radical scavenging activity. AYH (1:100) and AYH (3:100) had higher reducing activities of 0.42 and 0.23, respectively at a concentration of 1000 µg/ml. -

Regulation of Energy Substrate Metabolism in Endurance Exercise
International Journal of Environmental Research and Public Health Review Regulation of Energy Substrate Metabolism in Endurance Exercise Abdullah F. Alghannam 1,* , Mazen M. Ghaith 2 and Maha H. Alhussain 3 1 Lifestyle and Health Research Center, Health Sciences Research Center, Princess Nourah bInt. Abdulrahman University, Riyadh 84428, Saudi Arabia 2 Faculty of Applied Medical Sciences, Laboratory Medicine Department, Umm Al-Qura University, Al Abdeyah, Makkah 7607, Saudi Arabia; [email protected] 3 Department of Food Science and Nutrition, College of Food and Agriculture Sciences, King Saud University, Riyadh 11451, Saudi Arabia; [email protected] * Correspondence: [email protected] Abstract: The human body requires energy to function. Adenosine triphosphate (ATP) is the cellular currency for energy-requiring processes including mechanical work (i.e., exercise). ATP used by the cells is ultimately derived from the catabolism of energy substrate molecules—carbohydrates, fat, and protein. In prolonged moderate to high-intensity exercise, there is a delicate interplay between carbohydrate and fat metabolism, and this bioenergetic process is tightly regulated by numerous physiological, nutritional, and environmental factors such as exercise intensity and du- ration, body mass and feeding state. Carbohydrate metabolism is of critical importance during prolonged endurance-type exercise, reflecting the physiological need to regulate glucose homeostasis, assuring optimal glycogen storage, proper muscle fuelling, and delaying the onset of fatigue. Fat metabolism represents a sustainable source of energy to meet energy demands and preserve the ‘limited’ carbohydrate stores. Coordinated neural, hormonal and circulatory events occur during prolonged endurance-type exercise, facilitating the delivery of fatty acids from adipose tissue to the Citation: Alghannam, A.F.; Ghaith, working muscle for oxidation. -

Photoremovable Protecting Groups Used for the Caging of Biomolecules
1 1 Photoremovable Protecting Groups Used for the Caging of Biomolecules 1.1 2-Nitrobenzyl and 7-Nitroindoline Derivatives John E.T. Corrie 1.1.1 Introduction 1.1.1.1 Preamble and Scope of the Review This chapter covers developments with 2-nitrobenzyl (and substituted variants) and 7-nitroindoline caging groups over the decade from 1993, when the author last reviewed the topic [1]. Other reviews covered parts of the field at a similar date [2, 3], and more recent coverage is also available [4–6]. This chapter is not an exhaustive review of every instance of the subject cages, and its principal focus is on the chemistry of synthesis and photocleavage. Applications of indi- vidual compounds are only briefly discussed, usually when needed to put the work into context. The balance between the two cage types is heavily slanted to- ward the 2-nitrobenzyls, since work with 7-nitroindoline cages dates essentially from 1999 (see Section 1.1.3.2), while the 2-nitrobenzyl type has been in use for 25 years, from the introduction of caged ATP 1 (Scheme 1.1.1) by Kaplan and co-workers [7] in 1978. Scheme 1.1.1 Overall photolysis reaction of NPE-caged ATP 1. Dynamic Studies in Biology. Edited by M. Goeldner, R. Givens Copyright © 2005 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 3-527-30783-4 2 1 Photoremovable Protecting Groups Used for the Caging of Biomolecules 1.1.1.2 Historical Perspective The pioneering work of Kaplan et al. [7], although preceded by other examples of 2-nitrobenzyl photolysis in synthetic organic chemistry, was the first to apply this to a biological problem, the erythrocytic Na:K ion pump. -

Two-Photon Excitation Fluorescence Microscopy
P1: FhN/ftt P2: FhN July 10, 2000 11:18 Annual Reviews AR106-15 Annu. Rev. Biomed. Eng. 2000. 02:399–429 Copyright c 2000 by Annual Reviews. All rights reserved TWO-PHOTON EXCITATION FLUORESCENCE MICROSCOPY PeterT.C.So1,ChenY.Dong1, Barry R. Masters2, and Keith M. Berland3 1Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139; e-mail: [email protected] 2Department of Ophthalmology, University of Bern, Bern, Switzerland 3Department of Physics, Emory University, Atlanta, Georgia 30322 Key Words multiphoton, fluorescence spectroscopy, single molecule, functional imaging, tissue imaging ■ Abstract Two-photon fluorescence microscopy is one of the most important re- cent inventions in biological imaging. This technology enables noninvasive study of biological specimens in three dimensions with submicrometer resolution. Two-photon excitation of fluorophores results from the simultaneous absorption of two photons. This excitation process has a number of unique advantages, such as reduced specimen photodamage and enhanced penetration depth. It also produces higher-contrast im- ages and is a novel method to trigger localized photochemical reactions. Two-photon microscopy continues to find an increasing number of applications in biology and medicine. CONTENTS INTRODUCTION ................................................ 400 HISTORICAL REVIEW OF TWO-PHOTON MICROSCOPY TECHNOLOGY ...401 BASIC PRINCIPLES OF TWO-PHOTON MICROSCOPY ..................402 Physical Basis for Two-Photon Excitation ............................ -

Spring 2013 Lecture 13-14
CHM333 LECTURE 13 – 14: 2/13 – 15/13 SPRING 2013 Professor Christine Hrycyna INTRODUCTION TO ENZYMES • Enzymes are usually proteins (some RNA) • In general, names end with suffix “ase” • Enzymes are catalysts – increase the rate of a reaction – not consumed by the reaction – act repeatedly to increase the rate of reactions – Enzymes are often very “specific” – promote only 1 particular reaction – Reactants also called “substrates” of enzyme catalyst rate enhancement non-enzymatic (Pd) 102-104 fold enzymatic up to 1020 fold • How much is 1020 fold? catalyst time for reaction yes 1 second no 3 x 1012 years • 3 x 1012 years is 500 times the age of the earth! Carbonic Anhydrase Tissues ! + CO2 +H2O HCO3− +H "Lungs and Kidney 107 rate enhancement Facilitates the transfer of carbon dioxide from tissues to blood and from blood to alveolar air Many enzyme names end in –ase 89 CHM333 LECTURE 13 – 14: 2/13 – 15/13 SPRING 2013 Professor Christine Hrycyna Why Enzymes? • Accelerate and control the rates of vitally important biochemical reactions • Greater reaction specificity • Milder reaction conditions • Capacity for regulation • Enzymes are the agents of metabolic function. • Metabolites have many potential pathways • Enzymes make the desired one most favorable • Enzymes are necessary for life to exist – otherwise reactions would occur too slowly for a metabolizing organis • Enzymes DO NOT change the equilibrium constant of a reaction (accelerates the rates of the forward and reverse reactions equally) • Enzymes DO NOT alter the standard free energy change, (ΔG°) of a reaction 1. ΔG° = amount of energy consumed or liberated in the reaction 2.